Volume 30, Number 12—December 2024
Research
Clinical Manifestations, Antifungal Drug Susceptibility, and Treatment Outcomes for Emerging Zoonotic Cutaneous Sporotrichosis, Thailand
Cite This Article
Citation for Media
Abstract
We analyzed clinical manifestations, antifungal susceptibility, and treatment outcomes of cutaneous sporotrichosis in Thailand during 2018–2022. The study included 49 patients whose mean age was 58.7 (SD 16.9) years; 65.3% were female and 34.7% male. A history of cat exposure was reported in 32 (65.3%) patients who had a significantly higher prevalence of upper extremity lesions than did those without cat contact (90.6% vs. 41.7%; adjusted odds ratio 18.9 [95% CI 3.2–92.9]). Among patients >60 years of age, lesions were more likely to be nonpustular than for patients <60 years of age (82.1% vs. 52.4%; p = 0.033). All 9 isolates tested for antifungal drug susceptibility exhibited an itraconazole MIC of <1 μg/mL. Oral itraconazole monotherapy was effective; the median time-to-cure was 180 days (interquartile range 141–240 days). Physicians should heighten their awareness of potential sporotrichosis causes, particularly when a history of animal contact exists.
Sporotrichosis is a chronic infection caused by Sporothrix spp., dimorphic fungi that exist in a hyphal form at low temperatures or as budding yeast at high temperatures. This pathogen is found in soil, plants, and organic matter and gives rise to an anthropozoonotic disease primarily affecting the skin and subcutaneous tissues after traumatic inoculation by contaminated materials (1–3). Sporothrix spp. have been previously classified under the S. schenckii complex, which comprise S. schenckii sensu stricto, S. brasiliensis, S. globosa, S. luriei, S. pallida, S. mexicana, and S. chilensis. The classification system has been updated to include a clinical clade consisting of S. schenckii, S. globosa, S. brasiliensis, and S. luriei. Species from the environmental clade, including S. pallida, S. mexicana, and S. chilensis, rarely cause human infection (4).
Although sporotrichosis has a global distribution, clinical and epidemiologic features vary. It is endemic in regions that have tropical and subtropical climates and maintain high humidity and 25°C–28°C temperatures (2,5,6). Several reports have cited case series or outbreak instances among occupational groups prone to minor soil- and plant-inflicted injuries, such as florists, miners, foresters, and gardeners (7–12). Furthermore, since the 1980s, owning cats has emerged as a risk factor for zoonotic transmission of sporotrichosis (13,14). Human sporotrichosis outbreaks have been linked to scratches or bites from infected cats. Domestic cats, because of their pathogen-rich lesions and intimate interactions with humans, serve as Sporothrix spp. vectors (15–20).
The disease has an incubation period ranging from a few days to several months (21) and can manifest in cutaneous, pulmonary, and disseminated forms. Cutaneous manifestation is the most common, typically occurring as erythematous papules or pustules that form ulcerated nodules involving local lymphatic channels, which cause lymphangitis and lymphadenopathy (2,22). Sporotrichosis can also be categorized into fixed cutaneous, lymphocutaneous, and disseminated cutaneous forms. Although the fixed and lymphocutaneous forms are commonly observed, disseminated cutaneous sporotrichosis is rarely reported except in immunosuppressed patients (23). Fungi isolation is the standard for diagnosis; the first-line treatment is itraconazole (24). For antifungal susceptibility testing of Sporothrix spp., broth microdilution, as recommended by the Clinical and Laboratory Standards Institute (https://clsi.org/standards/products/microbiology/documents/m38), is the primary protocol used to determine in vitro MICs for drugs used in sporotrichosis treatment. Itraconazole is the most effective drug for treating sporotrichosis. A cutoff point of 1 μg/mL is used according to a previous study correlating the itraconazole MIC with clinical outcomes (25). Terbinafine has demonstrated the lowest geometric mean MIC, followed by ketoconazole (26). No associations have been found between higher amphotericin B or itraconazole MIC values and unfavorable outcomes (25).
Sporotrichosis is a relatively rare disease in Thailand. However, since 2018, a substantial increase in the number of sporotrichosis patients has been observed at the outpatient dermatology clinic at Siriraj Hospital in Bangkok, Thailand. This surge parallels the growing evidence of feline sporotrichosis observed in Thailand since 2018 (27). We investigated the clinical manifestations and treatment outcomes for a recent emerging outbreak of cutaneous sporotrichosis in Thailand.
We conducted a retrospective study of patients who had a cutaneous sporotrichosis diagnosis at the dermatology clinic of Siriraj Hospital during January 1, 2018–August 27, 2022. The Siriraj Hospital Institutional Review Board authorized the study protocol (approval nos. Si 979/2020 and Si 671/2021). We enrolled patients in the study if the diagnosis was confirmed by positive isolation of S. schenckii complex in culture. We extracted patient sex, age, duration of symptoms, underlying medical conditions, occupation, exposure history (animal contact, injury history, and contamination with soil), clinical manifestations, histopathology, laboratory culture, susceptibility testing of the causative fungus, treatment options, and treatment outcomes from medical records. We considered patients to be immunocompromised if they exhibited specific conditions that can cause host defense defects, such as cirrhosis, diabetes mellitus, or recent chemotherapy (28).
Hospital staff collected specimens for histopathologic examination and laboratory culture; specimens were collected from tissue biopsies or pus from cutaneous lesions. Fungi were identified by phenotypic characteristics, including colony morphology, conidial arrangement, and physiologic traits (carbohydrate assimilation of dextrose, sucrose, and raffinose), as well as temperature tolerance at 37°C and 40°C. Antifungal susceptibility testing was undertaken in some cases on the basis of convenience selection by using the Clinical and Laboratory Standards Institute broth dilution method, as noted . Testing was performed for 5-flucytosine, amphotericin B, anidulafungin, caspofungin, micafungin, fluconazole, itraconazole, posaconazole, and voriconazole.
We defined an improved lesion as a lesion that had become smaller or drier without cutaneous extension and a cure as a complete healing of lesions with or without scarring or hyperpigmentation. We conducted telephone interviews to assess patients who had incomplete data because of loss to follow-up during the treatment course.
Statistical Analysis
We used descriptive statistics for demographic data, clinical manifestations, histopathologic examinations, laboratory findings, antifungal susceptibility, treatments, and treatment outcomes. We calculated means and SDs for normally distributed continuous data, medians and interquartile ranges (IQRs) for non–normally distributed continuous data, and numbers and percentages for categorical data. We used χ2 and Fisher exact tests to compare clinical characteristics between patients who had or did not have exposure to cats. We used a binary logistic regression model and forward stepwise selection method to estimate the odds ratio (OR) of a binary response. We constructed a Cox proportional hazards model to determine the hazard ratio for each factor and to measure time-to-improve and time-to-cure. We adjusted for patient sex and age in the multivariable analysis of both binary logistic regression and Cox proportional hazards models. In addition, we calculated the OR, hazard ratio, and 95% CI for each pertinent variable. We analyzed data by using PASW Statistics 18.0 (SPSS, https://www.sbas.com.hk). A p value of <0.05 indicated statistical significance.
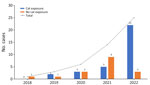
We included 49 patients who had cutaneous sporotrichosis during 2018–2022 and summarized their clinical characteristics (Table 1). We observed a marked increase in cases each year during this period, escalating from 1 case to 3 cases, then to 6 cases, 14 cases, and finally 25 cases (Figure 1). The mean age of patients was 58.7 (SD 16.9) years; 32 (65.3%) were female and 17 (34.7%) male. Only 9 patients were immunocompromised, having diabetes mellitus or sigmoid colon cancer with recent chemotherapy. The median duration from lesion onset to diagnosis was 30 (range 7–180) days. Before sporotrichosis diagnosis, 15 (30.6%) patients had undergone treatment regimens for nontuberculous mycobacterial infections but had no observed improvement.
Thirty-five (71.4%) patients reported a history of animal exposure; 32 had been exposed to cats and 3 to insects. Of the 3 patients with insect bites, 2 had bites from mosquitoes and 1 had an unidentified insect bite. For all 3 patients, lesions developed later on the lower extremities at the sites of those insect bites. Among the patients exposed to cats, 23 had direct contact, and 22 (95.7%) of those 23 reported scratches or bites (Table 2). Most (27/32) patients exposed to cats had contact with their own cats, and all let their cats roam outdoors. In addition, 21 (65%) patients had been in direct contact with cats that had sporotrichosis diagnosed by a veterinarian. Although 15 (30.6%) patients were retired or unemployed, 4 (8.2%) patients were employed in veterinary professions; 2 were veterinarians and 2 veterinary assistants. All 4 of those patients were scratched or bitten by cats that had sporotrichosis. Only 2/49 (4.1%) patients had a history of gardening, and none reported exposure to rose thorns. One patient reported falling from a bicycle onto soil causing an abrasion wound on his left leg, which later developed into lymphocutaneous lesions. Sporotrichosis developed in 13/49 (26.5%) patients who had no recognized risk factors.
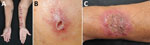
No pain was experienced in 49.0% of patients. Multiple lesions were observed in 59.2% of cases manifesting a lymphocutaneous pattern (44.9%; Figure 2, panel A). Fifteen (30.6%) patients had purulent discharge and ulceration. Single lesions, which mostly had satellite nodules around the ulcer rim, were found in 40.8% of patients (Figure 2, panel B). Three (6.1%) patients had solitary or multiple verrucous plaques (Figure 2, panel C). Most (73.5%) lesions were located on the upper extremities, and only 13.6% of patients manifested lymphadenopathy. Patients >60 years of age also had significantly more nonpustular lesions than younger patients (23/28 [82.1%] vs. 11/21 [52.4%]; p = 0.033).
Patients with a history of cat contact exhibited higher levels of immunocompetence (90.6% vs. 64.7%; adjusted OR 5.2 [95% CI 1.1–24.8]), and lesions were more frequently located on the upper extremities (90.6% vs. 41.7%; adjusted OR 18.9 [95% CI 3.2–92.9]) than in those who had no cat exposure (Table 3). The difference in lesion frequency was also statistically significant in multivariate analysis. In addition, lesions tended to be multiple and arranged in a lymphocutaneous pattern in patients who had cat contact.
Histopathologic analysis was conducted for 44 of 49 patients and revealed mixed cell or suppurative granuloma in 81.8% of lesions and nonspecific granulomatous inflammation in 6.8% of lesions. Periodic acid Schiff and Gomori methenamine silver stains were performed in all cases. No evidence of fungi was detected. Only 1 patient did not undergo a skin biopsy; their sporotrichosis diagnosis was confirmed through a positive pus culture result. Antifungal susceptibility testing for 9 patients was reported, and all 9 isolates exhibited an itraconazole MIC of <1 μg/mL (Table 4).
Of the 49 patients, 41 received itraconazole and 5 terbinafine as first-line treatment. The remaining 3 patients were lost to follow-up before beginning treatment. Treatment outcomes could only be observed in 41 patients; the remaining 8 were lost to follow-up. All itraconazole-treated patients received an initial dose of 200 mg/day. After ≈2 months of treatment, the itraconazole dose was increased to 400 mg/day in 11 (26.8%) patients because of worsening lesions. All 11 patients entered remission within a median of 127 (IQR 60–203) days after the dose increase. Antifungal susceptibility testing had been performed for 2 of those 11 patients (patients 2 and 7; Table 4), 1 of whom failed to improve after 4 months of increased itraconazole dose and was, therefore, treated by excision, followed by another 7 weeks of itraconazole and remission. We did not observe a significant difference in itraconazole MICs between patients who had treatment failure and those who had successful treatment at the dose of 200 mg/day (median MIC 1.0 [IQR 0.25–1.0] μg/mL vs. 0.5 [IQR 0.25–0.625] μg/mL; p = 0.414). No patients reported serious side effects from itraconazole.
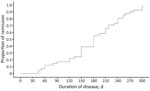
The median time-to-improvement was ≈46 (IQR 30–90) days, and the median time-to-cure was 180 (IQR 141–240) days. According to the Cox proportional hazards model, no variable was significantly associated with improvement or cure rates (Table 5). By 6 months after treatment, remission had been achieved in 50% of patients, and by 8 months, ≈75% of patients had entered remission (Figure 3).
Four human cases of sporotrichosis have previously been reported in Thailand (29–31). This study documents a high number of patients with sporotrichosis reported in the country during 2018–2022. The historically low reported incidence might have been from underreporting or underdiagnosis, possibly because of limited laboratory capabilities in Thailand (29).
Sporotrichosis is endemic in various regions worldwide (32), including Latin America, South Africa, Australia, and several countries in Asia, such as India, China, Japan, and Malaysia (33). The largest known zoonotic outbreak since the 20th Century was reported in Brazil (32). In Thailand, sporotrichosis cases have rarely been documented before 2018; our hospital typically encountered an average of only 1 case per year, initially linked to a case series reported in another hospital (29). However, since 2018, sporotrichosis has been increasingly reported in stray cats in Thailand (34), accompanied by a corresponding rise in human disease diagnosed at our hospital. Laboratory detection methods have remained consistent; conventional fungal detection techniques have been used routinely at our facility because of their cost-effectiveness for clinical services, whereas molecular techniques are primarily reserved for research purposes. Therefore, the increase in case numbers is more likely attributable to a true outbreak.
More women were enrolled in this study, ≈2 times more women than men. This ratio aligns with most other sporotrichosis outbreak reports, which have indicated similar percentages of women in study populations, ranging from 53% to 72% (35–38). The most commonly reported mean or median age has been 40–50 years (35–38), which is younger than that found in this study. Most patients were immunocompetent hosts, suggesting that immune status is not a determining factor for susceptibility to sporotrichosis.
A history of direct contact with cats that had current sporotrichosis diagnoses was reported by 21 (42.8%) of the 49 patients, suggesting that zoonotic transmission from cats was likely for those particular cases. Cats are the principal transmission vector leading to sporotrichosis outbreaks in many countries, particularly in South America and some countries in Asia (16,33,39). Claws, skin lesions, nasal and oral cavities, and feces of infected cats contain a considerably higher number of fungi than environmental sources, suggesting that Sporothrix spp. transmission more likely occurs from infected cats than from environmental sources (40). The rising incidence of the disease in humans aligns with the previously reported outbreak of feline sporotrichosis in Thailand (27). Although insects are known to be carriers of Sporothrix spp. fungi (41), a history of insect bites in patients with sporotrichosis has rarely been reported. In this study, 3 patients had lesions located in the area of previous insect bites. Sapronosis is another commonly cited primary mode of transmission in sporotrichosis outbreak studies (35). However, only 1 patient in this study reported possible sapronotic transmission from contact with contaminated soil after a bicycle fall.
The upper extremities have been ranked first and lower extremities ranked second as the most common sites for Sporothrix lesions (29,35,36). Moreover, lesions in the upper extremities are more likely to be reported in patients exposed to cats. Most sporotrichosis manifestations were lymphocutaneous or fixed cutaneous forms and occurred in similar proportions, aligning with our results. Disseminated sporotrichosis has rarely been reported globally (29,37,38) and has not been reported in Thailand (29).
Patients >60 years of age tended to have nonpustular lesions more frequently than did younger persons. This age-related pattern could be caused by immunosenescence, which is characterized by alterations in innate and adaptive immunity (42,43). As a person ages, their immunologic functions gradually decline, contributing to poor responses and diminished levels of inflammation after new infections (42,43).
Different Sporothrix species are predominant in various regions worldwide. S. schenckii is the primary species causing sporotrichosis in Australia, America, and South Africa, whereas S. globosa is the etiologic agent in most patients in China (4,24). S. brasiliensis is the main organism causing feline and human sporotrichosis in Brazil (4,44). Feline sporotrichosis caused by S. schenckii s.s. has been reported in southern Thailand, which suggests species transmission to humans (27). In Thailand, further studies and molecular identification of species causing human sporotrichosis are needed to explain the epidemiology and association with feline sporotrichosis. This study only reported the S. schenckii complex by using fungal culture and colony morphologic identification during routine laboratory investigations in clinical practice.
No evidence of fungal forms was seen in our cases during histopathologic examination; the absence of fungi in histopathologic sections remains unclear. However, on the basis of previous research, several factors might influence pathogen load in tissue biopsies. One factor is the onset or duration of the disease before the biopsy is obtained. A study of liver granuloma formation after Histoplasma sp. infection reported the highest fungal load in tissue at 7 days postinfection; substantial infection control was observed by 50 days (45). That finding suggests that early biopsy within the first week of symptoms might increase the likelihood of detecting the pathogen. Conversely, over time, granulomas might undergo structural changes that reduce their capacity to contain the infection (46). Another factor is the virulence of the pathogen; some fungi possess virulence factors that enable them to overcome host defenses (47). For example, S. brasiliensis has structural features that potentially inhibit and evade phagocytosis, contributing to its higher virulence than that for S. schenckii (48). Those factors might explain why our findings align with a study in Malaysia that reported low evidence of fungal organisms in tissues, rather than with higher detection rates observed in Brazil (38,39). Further studies involving larger populations are needed to explore those hypotheses.
Numerous treatments for cutaneous sporotrichosis are available. For example, oral antifungal agents, a saturated oral solution of potassium iodide, and excision can be used as standalone or combination therapies. However, oral itraconazole remains the treatment of choice, and the length of treatment is dependent upon lesion recovery (4,24,36). Despite the lack of a defined cutoff value for antifungal susceptibility among Sporothrix spp., our study found that all 9 isolates subjected to susceptibility testing exhibited an itraconazole MIC of <1 μg/mL. However, no significant difference was observed for itraconazole MICs between patients with and without treatment failure at a dose of 200 mg/day. Time-to-event analysis also showed no significant difference in time-to-improvement or time-to-cure between patients with a 1 μg/mL MIC and <1 μg/mL MIC, consistent with the study from Brazil (25). This study’s median duration until cure was 180 days, aligning with the findings from a 19-case review in Southeast Asia (29) and other studies (49,50). This study did not identify any clinical factors that influenced the duration until cure.
In conclusion, cutaneous sporotrichosis is a rare disease in Thailand. However, the number of cases has escalated dramatically since 2018, and ≈65% of patients have reported a history of cat exposure. Consequently, taking a thorough risk-factor history, especially regarding cat exposure, is crucial for guiding early clinical suspicion of disease and diagnosis. The standard for diagnosis is fungus isolation; the S. schenckii complex is suspected to be the primary sporotrichosis-causing species in Thailand. Itraconazole is the first-line treatment, and resistance to this drug has not been reported. Physicians and veterinary personnel should heighten their awareness of the sporotrichosis outbreak in Thailand to enable more effective disease control. In addition, early recognition of suspected sporotrichosis cases in cats, appropriate antifungal treatment, and education on how persons can avoid zoonotic transmission and manage owned or infected cats are all recommended to potentially minimize zoonotic transmission of sporotrichosis.
Dr. Jirawattanadon is a staff member in the Department of Dermatology, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand. Her research interests focus on dermatology and infectious diseases.
References
- Lopes-Bezerra LM, Schubach A, Costa RO. Sporothrix schenckii and sporotrichosis. An Acad Bras Cienc. 2006;78:293–308. DOIPubMedGoogle Scholar
- Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:
272376 . DOIPubMedGoogle Scholar - Rodrigues AM, Bagagli E, de Camargo ZP, Bosco SM. Sporothrix schenckii sensu stricto isolated from soil in an armadillo’s burrow. Mycopathologia. 2014;177:199–206. DOIPubMedGoogle Scholar
- Orofino-Costa R, Macedo PM, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–20. DOIPubMedGoogle Scholar
- Bonifaz A, Saúl A, Paredes-Solis V, Fierro L, Rosales A, Palacios C, et al. Sporotrichosis in childhood: clinical and therapeutic experience in 25 patients. Pediatr Dermatol. 2007;24:369–72. DOIPubMedGoogle Scholar
- Paixão AG, Galhardo MCG, Almeida-Paes R, Nunes EP, Gonçalves MLC, Chequer GL, et al. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res Ther. 2015;12:16. DOIPubMedGoogle Scholar
- Werner AH, Werner BE. Sporotrichosis in man and animal. Int J Dermatol. 1994;33:692–700. DOIPubMedGoogle Scholar
- Quintal D. Sporotrichosis infection on mines of the Witwatersrand. J Cutan Med Surg. 2000;4:51–4. DOIPubMedGoogle Scholar
- Centers for Disease Control (CDC). Multistate outbreak of sporotrichosis in seedling handlers, 1988. MMWR Morb Mortal Wkly Rep. 1988;37:652–3.PubMedGoogle Scholar
- Mahmoudi S, Zaini F. Sporotrichosis in Iran: A mini review of reported cases in patients suspected to cutaneous leishmaniasis. Curr Med Mycol. 2015;1:39–45. DOIPubMedGoogle Scholar
- Gremião IDF, Miranda LHM, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13:
e1006077 . DOIPubMedGoogle Scholar - Lyon GM, Zurita S, Casquero J, Holgado W, Guevara J, Brandt ME, et al.; Sporotrichosis in Peru Investigation Team. Population-based surveillance and a case-control study of risk factors for endemic lymphocutaneous sporotrichosis in Peru. Clin Infect Dis. 2003;36:34–9. DOIPubMedGoogle Scholar
- de Lima Barros MB, de Oliveira Schubach A, Galhardo MC, Schubach TM, dos Reis RS, Conceição MJ, et al. Sporotrichosis with widespread cutaneous lesions: report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. Int J Dermatol. 2003;42:677–81. DOIPubMedGoogle Scholar
- Barros MB, Schubach AO, do Valle AC, Gutierrez Galhardo MC, Conceição-Silva F, Schubach TM, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529–35. DOIPubMedGoogle Scholar
- Fleury RN, Taborda PR, Gupta AK, Fujita MS, Rosa PS, Weckwerth AC, et al. Zoonotic sporotrichosis. Transmission to humans by infected domestic cat scratching: report of four cases in São Paulo, Brazil. Int J Dermatol. 2001;40:318–22.PubMedGoogle Scholar
- Kovarik CL, Neyra E, Bustamante B. Evaluation of cats as the source of endemic sporotrichosis in Peru. Med Mycol. 2008;46:53–6. DOIPubMedGoogle Scholar
- Schubach AO, Schubach TM, Barros MB. Epidemic cat-transmitted sporotrichosis. N Engl J Med. 2005;353:1185–6. DOIPubMedGoogle Scholar
- Reis RS, Almeida-Paes R, Muniz MM, Tavares PM, Monteiro PC, Schubach TM, et al. Molecular characterisation of Sporothrix schenckii isolates from humans and cats involved in the sporotrichosis epidemic in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2009;104:769–74. DOIPubMedGoogle Scholar
- Mercurio MG, Elewski BE. Therapy of sporotrichosis. Semin Dermatol. 1993;12:285–9.PubMedGoogle Scholar
- Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24:633–54. DOIPubMedGoogle Scholar
- Freitas DFS, de Siqueira Hoagland B, do Valle ACF, Fraga BB, de Barros MB, de Oliveira Schubach A, et al. Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med Mycol. 2012;50:170–8. DOIPubMedGoogle Scholar
- Sizar O, Talati R. Sporotrichosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023
- Fichman V, Almeida-Silva F, Francis Saraiva Freitas D, Zancopé-Oliveira RM, Gutierrez-Galhardo MC, Almeida-Paes R. Severe sporotrichosis caused by Sporothrix brasiliensis: antifungal susceptibility and clinical outcomes. J Fungi (Basel). 2022;9:49. DOIPubMedGoogle Scholar
- Marimon R, Serena C, Gené J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732–4. DOIPubMedGoogle Scholar
- Indoung S, Chanchayanon B, Chaisut M, Buapeth KO, Morteh R, Jantrakajorn S. Feline sporotrichosis caused by Sporothrix schenckii sensu stricto in Southern Thailand: phenotypic characterization, molecular identification, and antifungal susceptibility. Med Mycol. 2022;60:
myac075 . DOIPubMedGoogle Scholar - Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. Microbiol Spectr. 2016;4:
4.4.43 . DOIPubMedGoogle Scholar - Tovikkai D, Maitrisathit W, Srisuttiyakorn C, Vanichanan J, Thammahong A, Suankratay C. Sporotrichosis: The case series in Thailand and literature review in Southeast Asia. Med Mycol Case Rep. 2020;27:59–63. DOIPubMedGoogle Scholar
- Kwangsukstith C, Vanittanakom N, Khanjanasthiti P, Uthammachai C. Cutaneous sporotrichosis in Thailand: first reported case. Mycoses. 1990;33:513–7. DOIPubMedGoogle Scholar
- Taninratapat N, Srisuttiyakorn C. Localized cutaneous sporotrichosis on face in healthy Thai female. Mycopathologia. 2019;184:539–42. DOIPubMedGoogle Scholar
- Queiroz-Telles F, Bonifaz A, Rossow J, Chindamporn A. Sporothrix and sporotrichosis. Encyclopedia Infect Immun. 2022;2:376–96. DOIGoogle Scholar
- Kamal Azam NK, Selvarajah GT, Santhanam J, Abdul Razak MF, Ginsapu SJ, James JE, et al. Molecular epidemiology of Sporothrix schenkii isolates in Malaysia. Med Mycol. 2020;58:617–25. DOIPubMedGoogle Scholar
- Yingchanakiat K, Limsivilai O, Sunpongsri S, Niyomtham W, Lugsomya K, Yurayart C. Phenotypic and genotypic characterization and antifungal susceptibility of Sporothrix schenckii sensu stricto isolated from a feline sporotrichosis outbreak in Bangkok, Thailand. J Fungi (Basel). 2023;9:590. DOIPubMedGoogle Scholar
- Sharma R, Mahajan VK, Singh Chauhan P, Mehta KS, Sharma A, Sharma J. The clinico-epidemiological characteristics and therapeutic experience of 152 patients with cutaneous sporotrichosis: a 10-year retrospective study from India. Int J Dermatol. 2021;60:99–106. DOIPubMedGoogle Scholar
- de Lima Barros MB, Schubach AO, de Vasconcellos Carvalhaes de Oliveira R, Martins EB, Teixeira JL, Wanke B. Treatment of cutaneous sporotrichosis with itraconazole—study of 645 patients. Clin Infect Dis. 2011;52:e200–6. DOIPubMedGoogle Scholar
- Brandolt TM, Madrid IM, Poester VR, Sanchotene KO, Basso RP, Klafke GB, et al. Human sporotrichosis: A zoonotic outbreak in southern Brazil, 2012-2017. Med Mycol. 2019;57:527–33. DOIPubMedGoogle Scholar
- Quintella LP, Passos SR, do Vale AC, Galhardo MC, Barros MB, Cuzzi T, et al. Histopathology of cutaneous sporotrichosis in Rio de Janeiro: a series of 119 consecutive cases. J Cutan Pathol. 2011;38:25–32. DOIPubMedGoogle Scholar
- Tang MM, Tang JJ, Gill P, Chang CC, Baba R. Cutaneous sporotrichosis: a six-year review of 19 cases in a tertiary referral center in Malaysia. Int J Dermatol. 2012;51:702–8. DOIPubMedGoogle Scholar
- Lloret A, Hartmann K, Pennisi MG, Ferrer L, Addie D, Belák S, et al. Sporotrichosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:619–23. DOIPubMedGoogle Scholar
- Carrada-Bravo T, Olvera-Macías MI. New observations on the ecology and epidemiology of Sporothrix schenckii and sporotrichosis 2. Ecological niches of S. schenckii and zoonotic outbreaks. Rev Mex Patol Clin Med Lab. 2013;60:5–24.
- Accardi G, Caruso C. Immune-inflammatory responses in the elderly: an update. Immun Ageing. 2018;15:11. DOIPubMedGoogle Scholar
- Fuentes E, Fuentes M, Alarcón M, Palomo I. Immune system dysfunction in the elderly. An Acad Bras Cienc. 2017;89:285–99. DOIPubMedGoogle Scholar
- Rodrigues AM, de Melo Teixeira M, de Hoog GS, Schubach TM, Pereira SA, Fernandes GF, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:
e2281 . DOIPubMedGoogle Scholar - Heninger E, Hogan LH, Karman J, Macvilay S, Hill B, Woods JP, et al. Characterization of the Histoplasma capsulatum-induced granuloma. J Immunol. 2006;177:3303–13. DOIPubMedGoogle Scholar
- Collette JR, Lorenz MC. Mechanisms of immune evasion in fungal pathogens. Curr Opin Microbiol. 2011;14:668–75. DOIPubMedGoogle Scholar
- Etchecopaz A, Toscanini MA, Gisbert A, Mas J, Scarpa M, Iovannitti CA, et al. Sporothrix brasiliensis: a review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J Fungi (Basel). 2021;7:170. DOIPubMedGoogle Scholar
- Restrepo A, Robledo J, Gómez I, Tabares AM, Gutiérrez R. Itraconazole therapy in lymphangitic and cutaneous sporotrichosis. Arch Dermatol. 1986;122:413–7. DOIPubMedGoogle Scholar
- Sharkey-Mathis PK, Kauffman CA, Graybill JR, Stevens DA, Hostetler JS, Cloud G, et al.; NIAID Mycoses Study Group. Treatment of sporotrichosis with itraconazole. Am J Med. 1993;95:279–85. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: November 15, 2024
Table of Contents – Volume 30, Number 12—December 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Penvadee Pattanaprichakul, Department of Dermatology, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wang Lang Rd, Bangkok Noi, Bangkok 10700, Thailand
Top