Volume 30, Number 2—February 2024
Research Letter
Integrating Veterinary Diagnostic Laboratories for Emergency Use Testing during Pandemics1
Figure
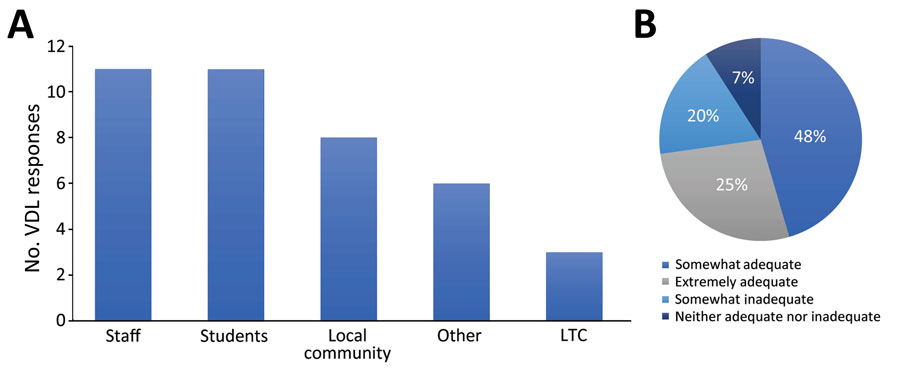
Figure. Population served (A) and perceived funding adequacy (B) of veterinary diagnostic laboratories (VDLs) conducting human SARS-CoV-2 testing, United States. A) Sum of responses for each of 5 selectable testing population types as reported by 13 of 17 VDLs performing human SARS-CoV-2 testing that responded to this optional question. VDLs could select any combination of answers that represented their specific testing populations. LTC, long-term care. B) Percentages of the 11 of 17 VDLs performing human SARS-CoV-2 testing that responded to the optional question to select 1 of 5 funding adequacy descriptions (no responses were received for inadequate).
1Preliminary results from this study were presented at the 65th American Association of Veterinary Laboratory Diagnosticians Conference and the 126th US Animal Health Association Annual Conference, October 6–12, 2022, Minneapolis, Minnesota, USA.
2These authors were co–principal investigators.