Volume 30, Number 2—February 2024
Dispatch
Introduction and Spread of Dengue Virus 3, Florida, USA, May 2022–April 2023
Cite This Article
Citation for Media
Abstract
During May 2022–April 2023, dengue virus serotype 3 was identified among 601 travel-associated and 61 locally acquired dengue cases in Florida, USA. All 203 sequenced genomes belonged to the same genotype III lineage and revealed potential transmission chains in which most locally acquired cases occurred shortly after introduction, with little sustained transmission.
Dengue virus (DENV) is not endemic in the continental United States (1); most cases occur among travelers to DENV-endemic areas (2). In Florida, USA, DENV infections are primarily reported among travelers (https://ndc.services.cdc.gov/case-definitions/dengue-virus-infections-2015); however, locally acquired cases and limited outbreaks have been reported in Monroe County in 2009–2010 (n = 88), Martin County in 2013 (n = 24), and Monroe County in 2020 (n = 72) (3–5). During 2009–2021, an annual median of 83 (range 19–413) travel-associated DENV infections and 7 (range 0–77) locally acquired cases were reported in Florida; all DENV types (DENV-1−4) occurred among both travel-associated and locally acquired cases (6). Previous work demonstrated the DENV vectors Aedes aegypti and A. albopictus mosquitoes are present across Florida (7).
In early 2022, the Florida Department of Health (FDOH) identified an increase in travel-associated DENV infections, primarily among travelers returning from Cuba. In July 2022, a DENV-3 outbreak was reported in Cuba (8); DENV-3 case increases were also documented in other countries in the Americas (9,10). On July 18, Miami-Dade County health officials issued a mosquito-borne illness advisory after the first locally acquired DENV infection in 2022 was confirmed in a Florida resident (11). We document the DENV-3 outbreak in Florida by describing the epidemiologic features of reported cases, analyzing DENV-3 genomic sequences, and reconstructing possible transmission trees.
FDOH routinely conducts active case-finding activities for DENV and conducts IgM and reverse transcription PCR testing for confirmation and DENV serotype identification. Suspected case-patients are interviewed to identify risk factors, possible mosquito exposure locations, and additional suspected cases (3). Ethics approval was not required because this work was part of standard public health outbreak surveillance and response.
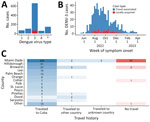
During May 1, 2022–April 30, 2023 (52 weeks), 1,037 DENV infections were reported, 966 (93%) were travel-associated and 71 (7%) locally acquired. DENV-3 was the most frequently identified serotype (64%, n = 662), followed by DENV-2 (10%, n = 104), DENV-1 (7%, n = 68), and DENV-4 (5%, n = 57); in 146 (14%) cases, multiple serotypes or no serotype was identified (Figure 1, panel A). Among DENV-3 cases, 601 (91%) were travel-associated and 61 (9%) were locally acquired cases (Figure 1, panel B). Most DENV-3 case-patients identified as White (n = 609; 92%) and Hispanic or Latino (n = 642, 97%).
Among 601 travel-associated DENV-3 cases, the median age was 52 (interquartile range 41–61) years; 51% of patients were male and 49% female. Most (98%, n = 589) case-patients with travel-associated DENV-3 had recently traveled from Cuba; they were reported in 21/67 Florida counties (Figure 1, panel C). Miami-Dade County had the most travel-associated DENV-3 cases (71%, n = 428). Among 61 locally acquired DENV-3 cases, the median age was 54 (interquartile range 36–58) years; 67% of patients were male and 33% female, and nearly all (93%, n = 57) were reported in Miami-Dade County. The 485 DENV-3 case-patients in Miami-Dade County were identified in 60/82 postal (ZIP) codes.
We performed genomic characterization of DENV-3 by sequencing the complete genomes of 203 cases at the Centers for Disease Control and Prevention (San Juan, Puerto Rico, USA), Yale School of Public Health (New Haven, CT, USA), and FDOH (Appendix 1) (12). Sequencing was prioritized and successful for 34 locally acquired cases, as well as case-patients with recent travel history to Cuba (n = 168) or Guyana (n = 1). To assess the representativeness of DENV sequences, we evaluated symptom onset dates and counties of residence for cases selected for sequencing and all cases detected (Appendix 1 Figure 1). We conducted maximum-likelihood phylogenetic analysis to infer the genetic relatedness of DENV-3 to contemporary circulation globally. Global context was provided with a subsample of 146 publicly available genomes that represent relevant genotypes.
The DENV-3 genomes identified in Florida are classified as genotype III and cluster within the novel American II lineage (9). We observed a close relationship with DENV-3 genomes recently identified in Arizona, Puerto Rico, Guyana, and Brazil, indicating that the lineage is spreading across the Americas (Figure 2). However, the limited sampling of the new American II lineage prevented us from inferring a potential time of emergence in Florida. The short branch lengths and similarity between locally acquired and travel-associated cases in the phylogenetic tree demonstrate low genomic diversity during the sampling period, where genomes from locally acquired cases cluster randomly with travel-associated cases. The tree topology suggests frequent importation events occurred during the sampling period and indicate frequent movement of DENV between Cuba and Florida without establishing sustained local transmission in Florida.
To model a possible transmission tree, we adapted a graph-based model using genomic sequences and symptom onset dates from 31 locally acquired and 144 travel-associated cases (Appendix 1) (13,14). To account for infections in transmission chains that went undetected between reported cases, we included a surveillance reporting probability (i.e., the probability an infection was detected as a case) and performed sensitivity analyses assuming different reporting probabilities of 1%, 5%, 10%, and 15%. Assuming a 5% reporting probability, we identified 22 travel-associated cases (15%) with most compatible linkages leading to the 31 locally acquired cases (Appendix 1 Figure 2). Overall, 122 (85%) travel-associated cases had no likely linkage to locally acquired cases, 17 (11%) were linked to 1 case, 2 (1%) were linked to 2 cases, 2 (1%) were linked to 3 cases, and 1 (1%) was linked to 4 cases.
We documented an unprecedented number of travel-associated and locally acquired DENV-3 cases in Florida during May 2022–April 2023; circulation of the DENV-3 genotype III was recently identified in the Americas. Our investigation illustrates that local transmission and spread in Florida was limited, despite multiple introductions from outside the country. Sequencing and phylogenetic analysis revealed that cases were from the same DENV-3 genotype III lineage and were highly related to one another and to cases identified in Puerto Rico, Arizona, and Brazil. Assessment of possible linkages between sequenced cases indicated that local transmission during this outbreak was limited; most travel-associated cases did not lead to further transmission.
DENV activity in Cuba and Florida are linked given their proximity and the extensive travel between them. Our results are similar to findings in Florida in 2019 (5), where many DENV case-patients reported recent travel to Cuba, leading to an increase in locally acquired cases. An elevated number of locally acquired DENV cases in Florida might be expected after a high number of introductions, but our analysis suggests that DENV introductions did not result in sustained local transmission beyond small-scale outbreaks. Factors potentially reducing transmission include living conditions (e.g., use of air conditioning and screens), rapid case notification that enabled vector interventions (e.g., spraying insecticide, conducting surveillance, community education, and removing standing water), or limited availability of mosquito breeding sites (15).
The relatively low genetic diversity in this dataset limited our ability to estimate the timing of initial DENV-3 introductions and fully reconstruct local spread. We did not use case locations to determine the compatibility of transmission links. DENV case detection continued through 2023 in Florida; efforts to understand those transmission dynamics are ongoing.
In summary, we used epidemiologic surveillance and genomic sequencing to identify a newly emerging lineage of DENV-3 genotype III that caused an unusually large number of travel-associated and locally acquired DENV infections in Florida, particularly in Miami-Dade County. Our analysis suggests that locally acquired cases were driven by large numbers of case-patients with recent travel to Cuba and that DENV persistence in Florida was limited. Close monitoring of DENV activity internationally, as well as increasing healthcare provider awareness about DENV identification and testing, can strengthen preparedness and response to future introductions in non–DENV-endemic areas.
Dr. Jones is an Epidemic Intelligence Service officer stationed at the Centers for Disease Control and Prevention Dengue Branch (Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases) in San Juan, Puerto Rico. His research interest is surveillance and modeling of infectious diseases.
Florida Department of Health Bureau of Public Health Laboratory Team: Sylvia Bunch, Natalia Cano, Amanda Davis, Yibo Dong, Rayah Jaber, Timothy Locksmith, Charles Panzera, Brittany Rowlette, Sarah Schmedes, Julieta Vergara.
Acknowledgments
We thank Joshua Wong for help with initial discussions on analysis plans. We also gratefully acknowledge the Arizona Department of Health Services and the Maricopa County Department of Public Health for contributing specimens for the phylogenetic analysis.
Code presented in this study is available at https://github.com/fjones2222/denv-3-florida-2022/.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number DP2AI176740 (NDG), and by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (CBFV), and by the National Institute of General Medical Sciences of the National Institutes of Health under award number R21GM142011 (SFM).
References
- Centers for Disease Control and Prevention. Dengue [cited 2023 Aug 19]. https://www.cdc.gov/dengue/index.html
- Wong JM, Rivera A, Volkman HR, Torres-Velasquez B, Rodriguez DM, Paz-Bailey G, et al. Travel-associated dengue cases—United States, 2010–2021. MMWR Morb Mortal Wkly Rep. 2023;72:821–6. DOIPubMedGoogle Scholar
- Rowe D, McDermott C, Veliz Y, Kerr A, Whiteside M, Coss M, et al.; Florida Department of Health Dengue Investigation Team. Dengue outbreak response during COVID-19 pandemic, Key Largo, Florida, USA, 2020. Emerg Infect Dis. 2023;29:1643–7. DOIPubMedGoogle Scholar
- Graham AS, Pruszynski CA, Hribar LJ, DeMay DJ, Tambasco AN, Hartley AE, et al. Mosquito-associated dengue virus, Key West, Florida, USA, 2010. Emerg Infect Dis. 2011;17:2074–5. DOIPubMedGoogle Scholar
- Sharp TM, Morris S, Morrison A, de Lima Corvino D, Santiago GA, Shieh WJ, et al.; 2019 Florida Dengue Investigation Team. Fatal Dengue Acquired in Florida. N Engl J Med. 2021;384:2257–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Historic data (2010–2022) [cited 2023 Aug 19]. https://www.cdc.gov/dengue/statistics-maps/historic-data.html
- Parker C, Ramirez D, Connelly CR. State-wide survey of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Florida. J Vector Ecol. 2019;44:210–5. DOIPubMedGoogle Scholar
- Embassy US. in Cuba. Health alert for U.S. citizens in Cuba on dengue fever [cited 2023 Aug 19]. https://cu.usembassy.gov/health-alert-for-u-s-citizens-in-cuba-on-dengue-fever
- Naveca FG, Santiago GA, Maito RM, Ribeiro Meneses CA, do Nascimento VA, de Souza VC, et al. Reemergence of dengue virus serotype 3, Brazil, 2023. Emerg Infect Dis. 2023;29:1482–4. DOIPubMedGoogle Scholar
- Kretschmer M, Collins J, Dale AP, Garrett B, Koski L, Zabel K, et al. Notes from the field: first evidence of locally acquired dengue virus infection—Maricopa County, Arizona, November 2022. MMWR Morb Mortal Wkly Rep. 2023;72:290–1. DOIPubMedGoogle Scholar
- Florida Health. Miami-Dade County. Health officials issue mosquito borne illness advisory following confirmation of one dengue case [cited 2023 Aug 19]. https://miamidade.floridahealth.gov/newsroom/2022/07/2022-07-18-mosquito-borne-illness-advisory.html
- Vogels C. DengueSeq: A pan-serotype whole genome amplicon sequencing protocol for dengue virus v1 [cited 2023 Sep 27]. https://www.protocols.io/view/dengueseq-a-pan-serotype-whole-genome-amplicon-seq-kqdg39xxeg25/v2
- Cori A, Nouvellet P, Garske T, Bourhy H, Nakouné E, Jombart T. A graph-based evidence synthesis approach to detecting outbreak clusters: An application to dog rabies. PLOS Comput Biol. 2018;14:
e1006554 . DOIPubMedGoogle Scholar - Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7:
e53 . DOIPubMedGoogle Scholar - Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–9. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: January 17, 2024
1These authors shared first authorship.
2Team members are listed at the end of this article.
Table of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Forrest Kirby Jones, Centers for Disease Control and Prevention, 1324 Calle Cañada, San Juan, 00920, Puerto Rico, USA
Top