Volume 30, Number 3—March 2024
Dispatch
Taenia martis Neurocysticercosis-Like Lesion in Child, Associated with Local Source, the Netherlands
Abstract
A neurocysticercosis-like lesion in an 11-year-old boy in the Netherlands was determined to be caused by the zoonotic Taenia martis tapeworm. Subsequent testing revealed that 15% of wild martens tested in that region were infected with T. martis tapeworms with 100% genetic similarity; thus, the infection source was most likely local.
The zoonotic Taenia martis tapeworm lives in mustelid intestines and has been reported across Europe (1). Human infection is thought to occur by accidental ingestion of eggs in mustelid feces and can lead to cysticercosis-like lesions, reported for only 6 adults in France, Germany, and Switzerland (2–7). We report a T. martis neurocysticercosis-like lesion in a child in the Netherlands.
In 2020, an 11-year-old boy was referred to the emergency department of University Medical Center Groningen (Gronigen, the Netherlands). Three days earlier, he had awakened with a frontal headache that intensified within 1 hour and led to nausea and vomiting. Symptoms resolved after sleep. On the evening of his referral to the emergency department, the boy suddenly became nauseous and pale, unable to speak, and in a decreased state of consciousness. His altered mental status continued for 20 minutes and his speech arrest for 90 minutes; a headache followed. No urine incontinence or tongue bite were noted. His medical history revealed only allergic rhinitis. He was an enthusiastic runner in the northern Netherlands woods and spent holidays in different nature areas of western Europe.
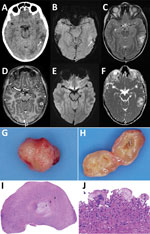
At the emergency department, his symptoms had resolved, and initial examination revealed no neurologic or laboratory test abnormalities. Computed tomography of his brain without contrast showed a hypodense area in the left temporal lobe and a barely discernable ringlike lesion with an isointense rim, without calcification (Figure 1, panel A). A cerebral venous sinus thrombosis was excluded. Magnetic resonance imaging (MRI) revealed a 13-mm round lesion with edema in the dorsal left temporal lobe and a hypointense rim on susceptibility-weighted and T2-weighted images (Figure 1, panels B, C), suggesting a fibrotic capsule, enhanced on 3-dimensional T1-weighted images (Figure 1, panel D). On diffusion-weighted images, no central diffusion restriction was seen (Figure 1, panels E, F). Initially, a brain tumor of undefined origin was proposed, but a second viewing suggested neurocysticercosis. Results of serologic testing of 2 samples collected 3 weeks apart, tested for T. solium tapeworms via a Centers for Disease Control and Prevention immunoblot recombinant antigen (rT24H antigen and LLGP) (8,9), were, however, negative.
The boy remained symptom free and because of the differential diagnosis of a brain tumor was referred to the national center for pediatric oncology in the Netherlands, the Princess Maxima Center (Utrecht, the Netherlands). Two weeks after the initial visit to University Medical Center Groningen, the patient underwent an uncomplicated craniotomy, and a cyst was extirpated in toto (Figure 1, panel G). Macroscopically, the lesion appeared to be an intact cystic round nodule on cut section with a white-greyish central area surrounded by a thin capsule (Figure 1, panel H). Microscopic examination revealed a necrotic core surrounded by fibrin and fibrosis (Figure 1, panel I) with adjacent multinuclear foreign body–type giant cells and an inflammatory infiltrate including plasma cells and eosinophilic neutrophils (Figure 1, panel J). No tegument or calcareous corpuscles were seen. After ruling out common pathogenic microorganisms, we determined that those features could fit well with the second (necrotic) stage of neurocysticercosis (10).
PCR analysis of the cyst material was performed by using the 12S rRNA gene as target (11) (primers: forward 5′-AAAIGGTTTGGCAGTGAGIGA-3′; reverse 5′GCGGTGTGTACITGAGITAAAC-3′) and with T. saginata DNA as positive control. PCR revealed a tapeworm infection, and sequencing indicated T. martis (Appendix). Those findings led to the final diagnosis of a stage 2 neurocysicercosis-like lesion, based on the T. martis infection. The patient received albendazole (2×/d for 1 week). Follow-up MRI of the brain 1.5 months after surgery showed only the resection cavity, and the boy has remained symptom free.
To explore potential sources, we investigated stone martens (Martes foina) that had been killed as part of ongoing predator control in 2020 and 2021 in Friesland, a northern province of the Netherlands. We checked their intestines macroscopically for T. martis tapeworms and collected intestinal content from multiple parts of the intestine to submit for molecular detection of T. martis tapeworm DNA. We extracted DNA from collected tapeworms and all intestinal scrapings by using the DNeasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com). We also performed conventional PCR targeting the CO1 gene on the patient material, using primers previously reported (12), with slight modification of the primers (forward, 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′; reverse, 5′-TAACGACATAACATAATGAAAATG-3′), followed by electrophoresis using 1.8% agarose gel PCR. We sequenced samples with bands matching the positive control, obtained from a T. martis worm collected at the start of the project, by using BaseClear (Leiden, https://www.baseclear.com) and performed BLAST analysis (https://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
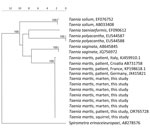
Of the 214 collected stone martens, sequences of 32 (15%, 95% CI 10%–20%) intestinal scraping samples matched T. martis sequences from GenBank, including samples from 7 stone martens in which adult tapeworms were macroscopically detected and confirmed by PCR and sequencing to be T. martis. Genetic analysis showed 100% similarity between the T. martis sequence of the patient (GenBank accession no. OR765728) and those from the martens. In addition, a T. martis sequence from larval cestode from a squirrel collected in 2014 in the Netherlands (provided by Herman Cremers) was 100% identical to the sequence from the patient. Sequences from T. martis tapeworms collected in Switzerland, Croatia, France, and Germany were 100% identical and from Italy 99.6% identical to the sequences of the martens from the Netherlands (Figure 2).
To our knowledge, human T. martis cysticercosis has been reported for only 6 adults. Two cases involved a T. martis neurocysticercosis-like lesion (2,7), and the others involved the eye, peritoneum, and pouch of Douglas (3–6). All 6 patients were immunocompetent women: 5 tended and ate from vegetable gardens, 5 lived in rural areas, and 3 were frequent hikers/dog owners. The boy we report also spent a lot of time in the forest.
Stone martens are synanthropic mustelids and will eat fruit or scavenge scraps from compost heaps in gardens and barnyards. It is hypothesized that consuming contaminated vegetables or fruit or accidentally ingesting T. martis eggs after contact with contaminated soil may lead to (neuro)cysticercosis-like infection caused by T. martis tapeworms.
Neurocysticercosis involves infection of the central nervous system by the larval stage of the pork tapeworm T. solium (13). The MRI features for the boy with a T. martis neurocysticercosis-like lesion and the patient in France resemble those caused by T. solium tapeworms (2). Features depend on stage of the infection (14). No specific serologic test is available for T. martis infection, and the extent of cross-reactivity between T. solium and T. martis antibodies in available serology tests is unknown. Serologic test results for the 6 adult patients showed mixed signals, including positive signals against Echinococcus multilocularis crude larval antigen extract (that could not be repeated in confirmatory assays) (5) and T. solium (2,5), although others have reported negative serologic test results for those parasites (3,4). Confirming the diagnosis requires detecting parasite DNA by PCR and sequencing to differentiate between Taenia species. The availability of differentiating molecular methods may have resulted in increased diagnoses of T. martis infections, possibly previously misdiagnosed as T. solium infections (3).
The finding of T. martis tapeworms in the patient and the stone martens we investigated from the northern part of the Netherlands strongly suggest a local source of infection. Although the prevalence of T. martis tapeworms can vary widely regionally (15), studies in host and reservoir species suggest widespread appearance of T. martis tapeworm in mustelids in Europe (1), and underrecognition and underreporting of cysticercosis caused by infection with this tapeworm is probable.
Dr. Eggink is a neurology resident at the University Medical Center Groningen, specializing in pediatric neurology and movement disorders. She combines her residency with a postdoctoral position focusing on recognizing pediatric movement disorders at the Movement Disorders Groningen Expertise Center.
Acknowledgment
The diagnosis in this case was the result of a collaboration between different centers within the Netherlands. We thank contributors Bob Jonge Poerink and Bob van den Brink for their help collecting the stone marten samples; Nahid Nozari and Cecile Dam-Deisz for their help performing the molecular assays for the patient and the martens; Maarten Heuvelmans and Tjomme van de Bruggen for their help finding the diagnosis; Joke van der Giessen for her help with the molecular analyses; and Herman Cremers for his help providing us with the T. martis tapeworms from the squirrel from his extensive collection of wildlife parasites.
References
- Deplazes P, Eichenberger RM, Grimm F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int J Parasitol Parasites Wildl. 2019;9:342–58. DOIPubMedGoogle Scholar
- Brunet J, Benoilid A, Kremer S, Dalvit C, Lefebvre N, Hansmann Y, et al. First case of human cerebral Taenia martis cysticercosis. J Clin Microbiol. 2015;53:2756–9. DOIPubMedGoogle Scholar
- Eberwein P, Haeupler A, Kuepper F, Wagner D, Kern WV, Muntau B, et al. Human infection with marten tapeworm. Emerg Infect Dis. 2013;19:1152–4. DOIPubMedGoogle Scholar
- Koch T, Schoen C, Muntau B, Addo M, Ostertag H, Wiechens B, et al. Molecular diagnosis of human Taenia martis eye infection. Am J Trop Med Hyg. 2016;94:1055–7. DOIPubMedGoogle Scholar
- Mueller A, Förch G, Zustin J, Muntau B, Schuldt G, Tappe D. Case report: molecular identification of larval Taenia martis infection in the pouch of Douglas. Am J Trop Med Hyg. 2020;103:2315–7. DOIPubMedGoogle Scholar
- Rudelius M, Brehm K, Poelcher M, Spinner C, Rosenwald A, da Costa CP. First case of human peritoneal cysticercosis mimicking peritoneal carcinosis: necessity of laparoscopy and histologic assessment for the correct diagnosis. JMM Case Rep. 2017;4:
e005097 . DOIPubMedGoogle Scholar - Steinsiepe VK, Ruf M-T, Rossi M, Fricker-Feer C, Kolenc D, Buser BS, et al. Human Taenia martis neurocysticercosis, Switzerland. Emerg Infect Dis. 2023;29:2569–72. DOIPubMedGoogle Scholar
- Gómez-Morales MÁ, Pezzotti P, Ludovisi A, Boufana B, Dorny P, Kortbeek T, et al. Collaborative studies for the detection of Taenia spp. infections in humans within CYSTINET, the European Network on Taeniosis/Cysticercosis. Microorganisms. 2021;9:1173. DOIPubMedGoogle Scholar
- Noh J, Rodriguez S, Lee Y-M, Handali S, Gonzalez AE, Gilman RH, et al. Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol. 2014;52:1429–34. DOIPubMedGoogle Scholar
- Pittella JEH. Pathology of CNS parasitic infections. Handb Clin Neurol. 2013;114:65–88. DOIPubMedGoogle Scholar
- Roelfsema JH, Nozari N, Pinelli E, Kortbeek LM. Novel PCRs for differential diagnosis of cestodes. Exp Parasitol. 2016;161:20–6. DOIPubMedGoogle Scholar
- Casulli A, Manfredi MT, La Rosa G, Cerbo AR, Genchi C, Pozio E. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet Parasitol. 2008;155:168–72. DOIPubMedGoogle Scholar
- Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–15. DOIPubMedGoogle Scholar
- Lerner A, Shiroishi MS, Zee C-S, Law M, Go JL. Imaging of neurocysticercosis. Neuroimaging Clin N Am. 2012;22:659–76. DOIPubMedGoogle Scholar
- Borgsteede FH, Tibben JH, van der Giessen JW. The musk rat (Ondatra zibethicus) as intermediate host of cestodes in the Netherlands. Vet Parasitol. 2003;117:29–36. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: February 01, 2024
1These first authors contributed equally to this article.
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Miriam Maas, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Antonie van Leeuwenhoeklaan 9, 3721 MA Bilthoven, the Netherlands
Top