Volume 30, Number 3—March 2024
Dispatch
Biphasic MERS-CoV Incidence in Nomadic Dromedaries with Putative Transmission to Humans, Kenya, 2022–2023
Figure 2
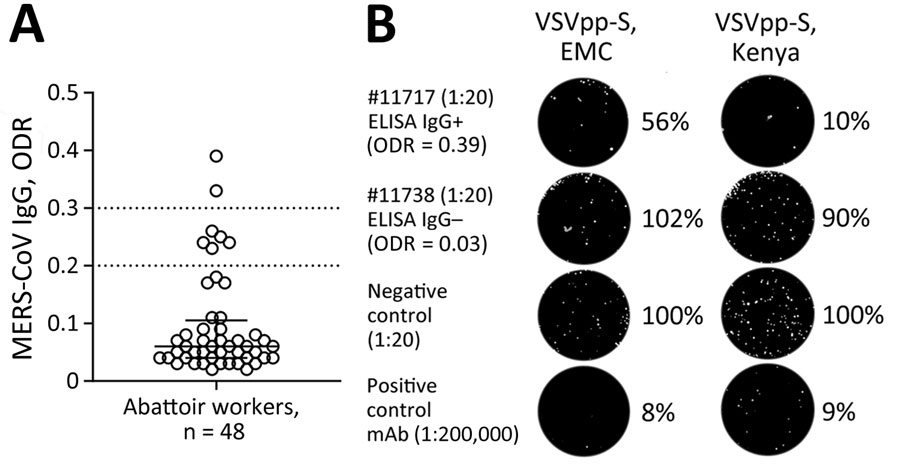
Figure 2. MERS-CoV immune responses in camel-exposed abattoir workers in Isiolo, Kenya. A) Results of commercial MERS-CoV S1-protein ELISA to detect IgG responses in 48 serum samples (diluted 1:100) from Isiolo abattoir workers. Samples with ODR >0.2 were considered ELISA-reactive, suggesting that 7/48 persons had MERS-CoV–reactive IgGs. Of note, all persons tested negative by MERS-CoV quantitative reverse transcription PCR. B) GFP-VSVpp-MERS-CoV S protein-based neutralization test (VSVpp-NT). VSVpp-S (EMC) and VSVpp-S (Kenya) contained human codon-optimized Spikes from prototypic MERS-CoV EMC/2012 clade A and Kenya clade C2.2 (#L00009980). All 7 ELISA-reactive human serum samples were mixed with 200 foci-forming units VSVpp in final serum dilutions 1:20–1:160. Out of the 7 ELISA-reactive persons, 1 showed a VSVpp-NT 50% foci-forming units reduction titer of 1:20 (EMC) and 1:40 (Kenya). The picture shows an example of the 1:20 dilution of an ELISA-reactive (#11717) and ELISA-nonreactive (#11738) abattoir worker. Negative control = ELISA-negative human serum (1:20) was used as reference and set to 100%. Positive control = monoclonal anti-MERS-CoV Spike receptor-binding domain binding antibody (mAb 7.7G6) previously shown to neutralize MERS-CoV at the tested dilution (1:2 × 105). For better graphical visibility, all pictures were enhanced in contrast and brightness identically. mAb, monoclonal antibody; MERS-CoV, Middle East respiratory syndrome coronavirus; ODR, optical density ratio; VSVpp, vesicular stomatitis virus pseudoparticles.
1These authors contributed equally to this article.