Volume 30, Number 4—April 2024
Dispatch
Novel Oral Poliovirus Vaccine 2 Safety Evaluation during Nationwide Supplemental Immunization Activity, Uganda, 2022
Figure
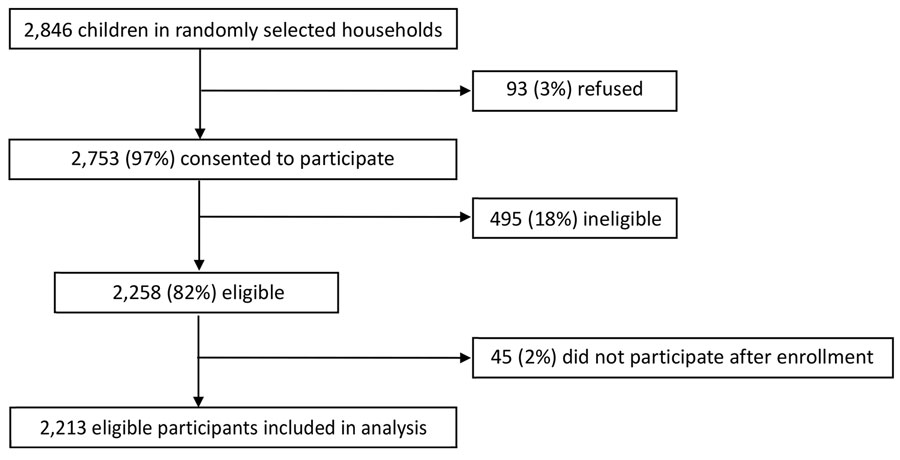
Figure. Cohort event monitoring enrollment after novel oral poliovirus vaccine type 2 administration, Uganda, 2022. Ineligible children included those who were >59 months of age, demonstrated acute signs or symptoms at the time of vaccination, were without a caretaker who had access to a phone, did not reside in the community for >42 days after vaccination, were without a caretaker staying with the child for >42 days, or did not complete enrollment, as well as any other unspecified reason.
Page created: February 08, 2024
Page updated: March 20, 2024
Page reviewed: March 20, 2024
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.