Volume 30, Number 4—April 2024
Research Letter
Drug-Resistant Tuberculosis, Georgia, Kazakhstan, Kyrgyzstan, Moldova, and Ukraine, 2017–2022
Cite This Article
Citation for Media
Abstract
In 2021, the World Health Organization recommended new extensively drug-resistant (XDR) and pre-XDR tuberculosis (TB) definitions. In a recent cohort of TB patients in Eastern Europe, we show that XDR TB as currently defined is associated with exceptionally poor treatment outcomes, considerably worse than for the former definition (31% vs. 54% treatment success).
In early 2021, the World Health Organization (WHO) recommended new definitions of extensively drug resistant (XDR) and pre-XDR tuberculosis (TB) (1,2). Previously, pre-XDR TB was informally defined as TB caused by Mycobacterium tuberculosis strains with resistance to rifampin and isoniazid plus resistance to either a fluoroquinolone or a second-line injectable, but not both (1–3). Now, pre-XDR TB is officially defined as strains with resistance to rifampin, isoniazid, and a fluoroquinolone (levofloxacin or moxifloxacin), whereas XDR TB is now defined as additional resistance to >1 group A drug (bedaquiline or linezolid), replacing the second-line injectables used in the former definitions (1–3).
Treatment outcomes of patients with XDR TB as currently defined have been sparsely reported. A study from France with 93 patients fulfilling the new pre-XDR TB and XDR TB definitions, including 9 patients with XDR TB, found a combined treatment success of 68% (n = 63), comparable to that of multidrug-resistant (MDR) TB (4). Another study following 9 XDR TB patients from Georgia documented a treatment success of only 22% (n = 2/9) (5). We describe MDR TB, pre-XDR TB, and XDR TB treatment outcomes in Georgia, Kazakhstan, Kyrgyzstan, Moldova, and Ukraine during 2017–2022 in patients with drug susceptibility tests available for fluroquinolones, second-line injectables, bedaquiline, and linezolid.
Using prospectively collected data from the National Institute of Allergy and Infectious Diseases TB Portals Program, as described elsewhere (6), we included 1,960 patients with MDR TB, pre-XDR TB, and XDR TB in the analysis. Median age was 43 (interquartile range 35–51) years; 78% (1,535) were men and 22% (425) women; 63% (1,235) were smokers, and 18% 350) were persons with HIV. Most patients were from Ukraine (n = 1,455), Moldova (n = 289), and Georgia (n = 160), whereas only a few were from Kazakhstan (n = 39) and Kyrgyzstan (n = 17).
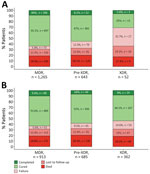
Of the 1,960 patients, 36% (698) were classified in a different category using the current definitions than for the previous definitions; XDR TB accounted for a much smaller percentage (2.7%, 95% CI 2.0%–3.5%) of patients than under the previous definition (18.5%, 95% CI 16.8%–20.3%). Using WHO treatment outcomes (6,7), our results showed that the current XDR TB definition was associated with low treatment success (sum of treatment completed and cured), only 31% (95% CI 19%–45%), compared with 54% (95% CI 49%–59%) using the former definition (p = 0.002 by χ2 test) (Figure). That finding was mainly driven by a higher percentage of failure using the current definition (33%, 95% CI 21%–47%) than when using the former (15%, 95% CI 11%–19%; p = 0.001) and a lower percentage of cured (25% [95% CI 14%–39%] vs. 46% [95% CI 41%–51%]; p = 0.004). Although a history of TB among patients with XDR TB was associated with a notably lower percentage of successful outcomes (23%, 95% CI 11%–41%) compared with the percentage of successful outcomes in persons without a history of TB (47%, 95% CI 24%–71%), this difference did not reach statistical significance (p = 0.076). MDR TB and pre-XDR TB treatment outcomes were comparable for both definitions.
Our study showed that XDR TB by the current definition is associated with exceptionally poor treatment outcomes, considerably worse than XDR TB by the former definition. The new XDR TB definition is applicable to fewer patients than the former definition, and treatment options are more limited. Veziris et al. (8) also observed a decrease in XDR TB patients using the revised definitions. Still, as use of bedaquiline and linezolid increases globally, resistance to those drugs will undoubtly increase, resulting in a higher number of XDR TB patients in the future. Better treatment outcomes for MDR TB have been associated with the use of bedaquiline, linezolid, and fluoroquinolones (2,9), and studies have shown worse outcomes for patients with bedaquiline resistance (10). Previous exposure to those drugs (i.e., bedaquiline, linezolid, fluoroquinolones) has been associated with worse patient outcomes compared with patients without previous exposure (5). Altogether, those factors explain the treatment success of only 31% for XDR TB, even lower than that recently found in a meta-analysis involving 10,223 XDR TB patients (94 studies, 26 countries) using the former definition, which together showed a pooled successful treatment outcome of 44% (95% CI 38%–50%) (3). That review found a XDR TB treatment success of 6% and 25% in 2 studies from Ukraine (n = 126).
The current definitions of pre-XDR TB and XDR TB, recommended since early 2021, are undoubtly more relevant than the former definitions, given they take into account WHO-recommended treatment regimens containing bedaquiline, pretomanid, linezolid, and moxifloxacin. Worryingly, but not surprisingly, the new definitions are associated with exceptionally poor outcomes for XDR TB, indicating loss of effective drugs. Upscaling of drug susceptibility testing, assessment of acquired drug resistance, and availability of diagnostic tools and drugs are crucial to avoid a future increase in patients with very limited treatment options. Treatment strategies should be assesed under programmatic conditions to improve understanding of the recommended treatment regimens, their implementation, and the effects on TB management globally.
Dr. Dahl is a medical doctor and PhD student at the Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark. He is interested in complicated respiratory infections such as tuberculosis and nontuberculous mycobacteria and mainly works with clinical epidemiology and systematic reviews.
Acknowledgment
This research was supported by the grants from the National Institute of Allergy and Infectious Diseases/US Civilian Research & Development Foundation, CRDF Global (DAA9-19-66199) and the Ministry of Health of Ukraine (#0123100178). The study funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
References
- World Health Organization. Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis [cited 2023 Dec 6]. https://www.who.int/publications/i/item/9789240018662
- Roelens M, Battista Migliori G, Rozanova L, Estill J, Campbell JR, Cegielski JP, et al. Evidence-based definition for extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2021;204:713–22. DOIPubMedGoogle Scholar
- Pedersen OS, Holmgaard FB, Mikkelsen MKD, Lange C, Sotgiu G, Lillebaek T, et al. Global treatment outcomes of extensively drug-resistant tuberculosis in adults: A systematic review and meta-analysis. J Infect. 2023;87:177–89. DOIPubMedGoogle Scholar
- Kherabi Y, Fréchet-Jachym M, Rioux C, Yazdanpanah Y, Méchaï F, Pourcher V, et al.; MDR-TB Management Group. Revised definitions of tuberculosis resistance and treatment outcomes, France, 2006–2019. Emerg Infect Dis. 2022;28:1796–804. DOIPubMedGoogle Scholar
- Mikiashvili L, Kempker RR, Chakhaia T, Bablishvili N, Avaliani Z, Lomtadze N, et al. Impact of prior TB treatment with new/companion drugs on clinical outcomes in patients receiving concomitant bedaquiline and delamanid for MDR/RR-TB. Clin Infect Dis. 2023;
ciad694 ; Epub ahead of print. DOIPubMedGoogle Scholar - Pedersen OS, Butova T, Kapustnyk V, Miasoiedov V, Kuzhko M, Hryshchuk L, et al. Treatment outcomes and risk factors for an unsuccessful outcome among patients with highly drug-resistant tuberculosis in Ukraine. Clin Microbiol Infect. 2024;30:360–7. DOIPubMedGoogle Scholar
- World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision: updated December 2014 and January 2020 [cited 2023 Dec 6]. https://www.who.int/publications/i/item/9789241505345
- Veziris N, Bonnet I, Morel F, Guglielmetti L, Maitre T, Fournier Le Ray L, et al.; CNR MyRMA; Members of the CNR-MyRMA (French National Reference Center for Mycobacteria. Impact of the revised definition of extensively drug-resistant tuberculosis. Eur Respir J. 2021;58:
2100641 . DOIPubMedGoogle Scholar - Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017; Ahmad N, Ahuja SD, Akkerman OW, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392:821–34.
- Ismail NA, Omar SV, Moultrie H, Bhyat Z, Conradie F, Enwerem M, et al. Assessment of epidemiological and genetic characteristics and clinical outcomes of resistance to bedaquiline in patients treated for rifampicin-resistant tuberculosis: a cross-sectional and longitudinal study. Lancet Infect Dis. 2022;22:496–506. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 12, 2024
Table of Contents – Volume 30, Number 4—April 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Victor Næstholt Dahl, Department of Infectious Diseases, Aarhus University Hospital, Palle Juul-Jensens Blvd 99, Aarhus, Denmark, DK-8200
Top