Volume 30, Number 7—July 2024
Research
Newly Recognized Spotted Fever Group Rickettsia as Cause of Severe Rocky Mountain Spotted Fever–Like Illness, Northern California, USA
Abstract
The incidence of spotted fever group (SFG) rickettsioses in the United States has tripled since 2010. Rocky Mountain spotted fever, the most severe SFG rickettsiosis, is caused by Rickettsia rickettsii. The lack of species-specific confirmatory testing obfuscates the relative contribution of R. rickettsii and other SFG Rickettsia to this increase. We report a newly recognized rickettsial pathogen, Rickettsia sp. CA6269, as the cause of severe Rocky Mountain spotted fever–like illness in 2 case-patients residing in northern California. Multilocus sequence typing supported the recognition of this pathogen as a novel Rickettsia genotype most closely related to R. rickettsii. Cross-reactivity observed for an established molecular diagnostic test indicated that Rickettsia sp. CA6269 might be misidentified as R. rickettsii. We developed a Rickettsia sp. CA6269–specific real-time PCR to help resolve this diagnostic challenge and better characterize the spectrum of clinical disease and ecologic epidemiology of this pathogen.
Rickettsioses are undifferentiated febrile illnesses, often accompanied by myalgia and rash, that are caused by intracellular gram-negative bacteria of the genus Rickettsia. Spotted fever group (SFG) Rickettsia are transmitted through the bite of ticks or mites and are grouped together on the basis of antigenic and genetic similarities that distinguish this group from other Rickettsia, namely the typhus group. The incidence of SFG rickettsioses in the United States increased 3-fold from 2010 to 2018 (1). The SFG Rickettsia associated with human illness in the United States include the tickborne agents R. rickettsii (Rocky Mountain spotted fever [RMSF]), R. parkeri (Rickettsia parkeri rickettsiosis), and Rickettsia 364D (Pacific Coast tick fever [PCTF]) and the miteborne agent R. akari (rickettsialpox) (1,2). Among the SFG rickettsioses, RMSF is the most severe; its case-fatality rate is 5%–10% in the United States (3,4). Unfortunately, the relative contribution of R. rickettsii and other rickettsial agents to the increase in SFG rickettsioses is largely unknown because of the reliance on group-specific (SFG or typhus group) serologic testing and the lack of species-specific confirmatory testing (1,5).
In California, SFG rickettsioses are a reportable condition tracked for public health surveillance purposes. California has the lowest incidence of SFG rickettsioses among reporting states and averages only 11 probable and 1 confirmed SFG rickettsiosis cases annually (1,6,7). Only RMSF and PCTF are endemic to California (7). Ticks infected with R. rickettsii are extremely rare in California; only 1 infected Dermacentor occidentalis tick and 2 Rhipicephalus sanguineus ticks have been detected despite numerous environmental surveys (7). In contrast, Rickettsia 364D, a genetic near neighbor of R. rickettsii, is found in 2.1% of adult D. occidentalis ticks (8,9). PCTF was recognized in 2008 and is clinically less severe than RMSF (10). The presence of an inoculation eschar at the site of a tick bite and lack of a rash can be useful in differentiating PCTF from RMSF (9). Another genetic near neighbor of R. rickettsii, Rickettsia sp. CA6269, recently was reported in rabbit ticks (Haemaphysalis leporispalustris) collected in Northern California (11). Multilocus sequence typing (MLST) of that strain revealed a novel genotype having sufficient sequence divergence from R. rickettsii to lead the investigators to propose a new species, Candidatus Rickettsia lanei. Here, we describe the clinical and epidemiologic features of severe RMSF-like illness in 2 patients in northern California who had disease onset dates separated by nearly 20 years and whose illness was caused by a newly identified SFG Rickettsia, Rickettsia sp. CA6269.
Clinical Specimens
Clinical specimens for confirmatory testing of suspected rickettsial diseases were submitted to the California Department of Public Health Viral and Rickettsial Disease Laboratory (CDPH-VRDL) for public health surveillance purposes and considered exempt from human subject regulations by the California Health and Human Services Agency Committee for the Protection of Human Subjects (project no. 2023-085). In addition to a plasma specimen from the initial case-patient, archival clinical specimens (serum or plasma) collected over the past 20 years from 8 confirmed SFG rickettsiosis case-patients were available for molecular characterization. SFG rickettsiosis cases were classified as confirmed for public health surveillance purposes on the basis of clinical and laboratory criteria (12).
Nucleic Acid Extraction and Real-time PCR
We extracted total nucleic acids using the NucliSENS easyMAG instrument (bioMérieux, https://www.biomerieux.com) with a serum or plasma input volume of 300 µL and a total nucleic acids output volume of 110 µL. We further concentrated total nucleic acids from case-patient 2 by 4-fold using the RNA Clean and Concentrator Kit (Zymo Research, https://www.zymoresearch.com).
We tested specimens for Rickettsia using a laboratory-developed triplex real-time reverse transcription PCR (rRT-PCR) targeting a R. rickettsii–specific 23S rRNA single-nucleotide polymorphism (SNP), a R. typhi–specific 23S rRNA SNP, and genus-specific regions of the 23S rRNA (Appendix). The assay was developed and the performance specifications established by the CDPH-VRDL as required under the Clinical Laboratory Improvement Amendments. A R. rickettsii–specific real-time PCR (rPCR) assay, RRi6, was performed as described by Kato et al. (13); the assay does not detect Rickettsia 364D (14).
MLST
We amplified and sequenced 6 target sequences, 23S rRNA, 16S rRNA, gltA, ompA, ompB, and sca4 (Appendix); the last 5 of those targets correspond to the regions recommended for the gene sequence–based classification of Rickettsia (15). A commercial laboratory performed bidirectional Sanger sequencing (ELIM Biopharmaceuticals, https://elimbio.com). We performed sequence editing and assembly using Geneious Prime 2022.0.2 (https://www.geneious.com). Nucleotide BLAST searches of the National Center for Biotechnology Information (NCBI) nucleotide collection and whole-genome shotgun contigs databases (https://blast.ncbi.nlm.nih.gov) were performed to identify genetic near neighbors and facilitate phylogenetic analyses. We concatenated and aligned the 16S rRNA, gltA, ompA, ompB, and sca4 sequences for validly named SFG Rickettsia species and constructed a maximum-likelihood phylogenetic tree using MEGA 10 (https://www.megasoftware.net). We measured the reliability of the resulting tree by bootstrap resampling using 1,000 replicates.
Tick Collection and Testing
We conducted field investigations at potential exposure sites by flagging vegetation and leaf litter for ticks. We identified tick species, sex, and life stages by examining morphologic features. For the 2023 case investigation, we processed adult ticks collected from Alameda and Contra Costa Counties for nucleic acid extraction using the DNeasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com). We screened tick nucleic acid extracts for R. rickettsii using the RRi6 assay. Ticks collected and identified from San Mateo and Marin Counties during the 2004 case investigation were screened for R. rickettsii DNA by the US Army Center for Health Promotion and Preventative Medicine–West.
Design and Development of a Rickettsia sp. CA6269 Real-Time PCR
We performed a nucleotide BLAST search using the ompA sequence from Rickettsia sp. CA6269 (GenBank accession no. JN990595) and downloaded and aligned selected sequences using the multiple sequence alignment tool. We selected regions of sequence divergence for primer and probe design using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast). We designed a 5′ exonuclease rPCR to amplify and detect a 146 bp region of ompA. The assay oligonucleotides were synthesized by Integrated DNA Technologies (https://www.idtdna.com). The 25-µL reaction mixture consisted of RLompAF1 (5′-GGGCACTTGGTGTTCCTACA-3′) and RLompAR1 (5′-AAATGCCCAATTGTTTTGAGGAC-3′) primers at 500 nM, RLompAP1 (6FAM- CTAATGGTG/ZEN/ATCCTACTGGCG-3IABkFQ) probe at 100 nM, 1X Premix ExTaq (Probe qPCR) mix (Takara Biosciences, https://www.takarabio.com), and 5 µL of total nucleic acids. We performed amplification and fluorescence detection with the ABI 7500 FAST DX Sequence Detection System (ThermoFisher Scientific, https://www.thermofisher.com) using the fast mode and the following amplification conditions: 1 minute at 95°C, 45 cycles of 95°C for 3 seconds, and 60°C for 30 seconds. We assessed assay analytical specificity by using a panel of 37 total nucleic acid extracts from members of the order Rickettsiales (Appendix) and evaluated analytical sensitivity by using 10-fold serial dilutions of a quantified 156-bp gBlock gene fragment (Integrated DNA Technologies) spiked into nucleic acids from pooled human blood and tested in replicates of five. We defined the assay limit of detection as the smallest number of DNA copies detected per reaction for all 5 replicates.
Case-Patient 1
The CDPH was notified of a suspected RMSF case involving a man with symptom onset in July 2023. The case-patient sought care at the emergency department (ED) with a 3-day history of influenza-like symptoms; he experienced fever, headaches, myalgias, arthralgias, malaise, loss of appetite, nonbloody diarrhea, and left-sided abdominal pain. Vital signs included temperature of 103°F (39.4°C), pulse of 96 beats/min, blood pressure of 116/71 mm Hg, and respiratory rate of 16 breaths/min. Lower left quadrant and suprapubic abdominal tenderness were noted upon physical examination. Of note, a rash was not observed. An antimicrobial regimen of ceftriaxone, metronidazole, and oral vancomycin was initiated, and the case-patient was hospitalized. The case-patient was transferred to the intensive care unit (ICU) on day 3 of hospitalization because of worsening hypoxemia, acidosis, encephalopathy, and seizures. Doxycycline was added to the treatment regimen on day 3 of hospitalization after an infectious diseases consultation that included rickettsial diseases in the differential diagnosis because of the severity of illness and clinical manifestations (Table 1).
Serologic testing for SFG Rickettsia by a commercial laboratory failed to detect a significant IgG titer in an acute serum specimen collected 5 days after symptom onset (Table 1). All blood cultures were negative. A laboratory diagnosis of rickettsial infection was initially made with the Karius Test, a microbial cell–free DNA (mcfDNA) metagenomic sequencing method (16). Sequencing of a plasma specimen collected 7 days after the onset of symptoms determined that R. rickettsii detected at 254,523 DNA molecules/microliter and R. slovaca detected at 97,653 DNA molecules/microliter were the best matches in the Karius Test genomic reference database. Serologic testing of a convalescent serum specimen, collected 105 days after symptom onset, demonstrated a significant IgG titer of >1:256 for SFG Rickettsia. The case-patient was discharged after being hospitalized for 22 days, including 11 days in the ICU, with a primary diagnosis of RMSF and secondary diagnoses of septic shock, acute kidney injury caused by acute tubular necrosis requiring intermittent hemodialysis, acute hypoxemic respiratory failure, encephalopathy, seizures, diarrhea, hyponatremia, abnormal liver enzymes, thrombocytopenia, supraventricular tachycardia, atrial fibrillation, and gangrene involving multiple digits on both hands.
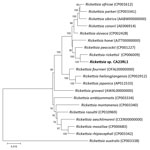
The residual plasma specimen from mcfDNA sequencing was provided to the CDPH-VRDL for species-specific confirmatory testing. We tested total nucleic acids extracted from this sample with the triplex rRT-PCR that detects a Rickettsia-specific sequence, an R. rickettsii–specific SNP, and an R. typhi–specific SNP in the 23S rRNA. Unexpectedly, only the Rickettsia species target, and not the R. rickettsii–specific SNP, was detected (cycle threshold [Ct] value 24.9) (Table 2). Subsequent testing with the RRi6 assay detected DNA at a Ct value of 30.5 (Table 2). To address the discordant R. rickettsii test results for the triplex rRT-PCR and the RRi6 assay, we sequenced a 1,468-nt segment of the rickettsial 23S rRNA (GenBank accession no. OR600926). Comparative sequence analysis revealed a 2-nt difference from Rickettsia 364D and a 3- to 4-nt difference from R. rickettsii, including an alternate SNP allele for the rRT-PCR SNP target. We further assessed species relatedness using a MLST scheme established for the classification of Rickettsia (15). We amplified and sequenced a 1,408 nt segment of 16S rRNA, a 1,051 nt segment of gltA, a 587 nt segment of ompA, a 4,849 nt segment of ompB, and a 2,944 nt segment of sca4 (GenBank accession nos. OR600927 and OR596747–50). Searches of the NCBI nucleotide databases revealed perfect sequence matches with gltA (290 nt) and sca4 (673 nt), a single nucleotide insertion/deletion difference in ompA (471 nt), and a single SNP difference in ompB (758 nt) from Rickettsia sp. CA6269 (GenBank accession nos. JN990594–7) (11). MLST results indicated that the strain, designated CA23RL1, was nearly identical to Rickettsia sp. CA6269. In contrast, CA23RL1 displayed significant sequence divergence from R. rickettsii and Rickettsia 364D at each MLST target (Table 3). Phylogenetic analysis of concatenated sequences from validly named SFG Rickettsia demonstrated that CA23RL1 occupied a distinct branch and was most closely related to R. rickettsii (Figure).
The case-patient had not traveled outside of the San Francisco Bay area within 19 days of illness onset and did not recall a tick bite. The case-patient had played golf at 5 courses located in Alameda and Contra Costa Counties within 14 days of symptom onset, including the day symptoms began. Of note, the case-patient recalled entering vegetation to retrieve golf balls at several courses. Because that activity might have been a potential source of exposure to ticks, as follow-up we collected ticks from the 5 golf courses within 16–22 days (late July and early August) of illness onset. In all, 197 adult ticks were collected and identified as either D. occidentalis (n = 135) or D. similis (n = 62). None of the ticks were infected with R. rickettsii or Rickettsia sp. CA6269 as determined by the RRi6 assay.
Case-Patient 2
We identified case-patient 2 through retrospective testing of 8 confirmed SFG rickettsiosis cases with the triplex rRT-PCR and RRi6 assays (Table 2). We detected Rickettsia spp. (Ct value 34.3), but not R. rickettsii or R. typhi, using the triplex rRT-PCR. The RRi6 assay detected rickettsial DNA (Ct value 34.3). Amplification and sequencing of a 397-nt segment of 23S rRNA (GenBank accession no. OR600925) by nested RT-PCR revealed an exact match to the 23S rRNA sequence determined for case-patient 1 and a 2- to 4-nt difference from Rickettsia 364D and R. rickettsii, supporting the identification of a second case of Rickettsia sp. CA6269 infection.
Case-patient 2, a male adult, was diagnosed with RMSF in 2004 on the basis of clinical features and a 4-fold increase in serologic titer to SFG Rickettsia in serum specimens collected 10 days apart (Table 1). Illness onset occurred in June; the case-patient sought care at the ED with a 5-day history of headaches, vomiting, photophobia, neck pain, and confusion. Upon initial examination, vital signs included a body temperature of 101.8°F (38.8°C), pulse of 96 beats/min, blood pressure of 143/89 mm Hg, and respiratory rate of 20 breaths/min. Physical examination revealed mild nuchal rigidity, a maculopapular rash on the arms and legs, and leg edema. The case-patient was hospitalized on the basis of the ED assessment that included encephalitis, sepsis, hypoxemia, rash of unknown etiology, and chronic leg edema, and antimicrobial treatment with ceftriaxone, vancomycin, and acyclovir was initiated. On day 3 of hospitalization, the patient became hypoxic and comatose and was intubated and transferred to the ICU for 4 days. Doxycycline was added to the treatment regimen on day 3 of hospitalization after an infectious diseases consultation that included rickettsioses in the differential diagnosis on the basis of clinical manifestations and the patient’s outdoor activities. Results of blood, CSF, urine, and sputum cultures were negative for significant pathogens. Serologic test results on day 10 of hospitalization indicated an IgG titer of 1:4,096 for SFG Rickettsia. After 13 days, the patient was discharged with a primary diagnosis of Rickettsia encephalitis.
The case-patient had not traveled outside the San Francisco Bay area during the 2 weeks before onset but had visited and camped at a county park and state beach in San Mateo County and a rural community in Marin County within that period. At the county park, he recalled finding a tick crawling on his body but not a tick bite. Field investigations at these locations conducted 30–40 days (July) after illness onset yielded 10 D. occidentalis ticks, 8 D. similis ticks, and 6 Ixodes pacificus ticks. Molecular testing of the Dermacentor spp. ticks did not detect R. rickettsii.
Rickettsia sp. CA6269–Specific Real-time PCR
After the sequence-based identification of Rickettsia sp. CA6269 infections, we developed an rPCR targeting genotype-specific regions of ompA. We did not observe assay cross-reactivity with nucleic acids from Anaplasma phagocytophilum, Ehrlichia chaffeensis, Orientia tsutsugamushi, and 14 Rickettsia species, including 10 R. rickettsii strains and 11 Rickettsia 364D strains (Appendix). The assay limit of detection was 1 copy of DNA per reaction. Of 9 SFG rickettsiosis case-patients tested, Rickettsia sp. CA6269 was detected only for case-patient 1 (Ct value 29.6) and case-patient 2 (Ct value 34.4) (Table 2). The remaining 7 case-patients were confirmed R. rickettsii infections on the basis of detection of the species-specific 23S rRNA SNP with the triplex rRT-PCR.
We describe the clinical and epidemiologic features of 2 case-patients with RMSF-like illness attributed to a newly recognized rickettsial pathogen, Rickettsia sp. CA6269. The case-patients experienced severe clinical manifestations shared with RMSF, including acute kidney injury and respiratory failure, cutaneous necrosis and gangrene, and encephalitis. No unique clinical features were recognized in the 2 case-patients that would distinguish between infections caused by Rickettsia sp. CA6269 and R. rickettsii. Both patients likely acquired the infections locally during outdoor activities: in 1 case, golfing at courses with adjacent wildlands, and in the other case, visiting parks and camping. Although neither case-patient recalled being bitten by a tick, 1 case-patient had observed a tick crawling on his body. Nearly half of reported RMSF cases do not recall tick bites (4). These cases illustrate that clinicians should remain cognizant of SFG rickettsiosis in the differential diagnosis of undifferentiated febrile illnesses despite the lack of travel to areas of rickettsiosis endemicity and the absence of a recognized tick bite. Field investigations at locations of suspected exposure produced mostly Dermacentor spp. ticks and no H. leporispalustris ticks. Molecular testing of Dermacentor spp. ticks, established vectors of R. rickettsii, failed to detect R. rickettsii or Rickettsia sp. CA6269. Those potential locations of exposure will be the focus of future environmental investigations timed to better correlate with peak H. leporispalustris tick questing behavior.
Rickettsia sp. CA6269 was discovered during a survey of H. leporispalustris ticks collected in California for SFG Rickettsia (11). Of 234 ticks collected at the northern California site, 1 larval pool and 1 nymph produced a unique genotype, Rickettsia sp. CA6269. This unique genotype was not identified in the 179 ticks collected in southern California. The investigators proposed designating this novel Rickettsia as Candidatus R. lanei on the basis of results from an abbreviated MLST scheme. We have extended this work by sequencing the full-length regions originally proposed for the sequence-based classification of Rickettsia and established that Rickettsia sp. CA6269 meets the 2003 criteria for defining a new Rickettsia species (15). More recently, 2 genome sequence-based methods have been proposed for the classification of Rickettsia species (17,18). One method used genome sequence–based criteria aligned with the classic taxonomic analyses of bacteria to delineate Rickettsia into 9 species; nearly all SFG Rickettsia were classified as a single species (17). The other approach used genome sequence–based criteria that account for the unique phenotypic characteristics of rickettsiae and established phylogenetic relationships that were consistent with the current taxonomic classification of Rickettsia (18). That method displayed a strong correlation with MLST for Rickettsia classification. Despite the lack of updated consensus criteria for the taxonomic classification of Rickettsia species, delineating Rickettsia sp. CA6269 as a strain or subspecies of R. rickettsii or a new species will benefit from isolating and cultivating strains, describing phenotypic characteristics including ecologic epidemiology, and determining whole-genome sequences.
Additional environmental studies are needed to investigate the geographic distribution, potential vectors, prevalence of infection, and reservoir hosts of Rickettsia sp. CA6269. The H. leporispalustris tick has been shown to be a maintenance vector of R. rickettsii and is distributed throughout the Americas from Alaska to Argentina (19,20). Adult H. leporispalustris ticks feed on rabbits and hares, whereas the nymph and larval stages feed on ground-frequenting birds and small rodents (21). Humans are rarely bitten by H. leporispalustris, indicating a limited role for this tick in transmitting SFG rickettsioses (22). Several surveys for Rickettsia infections in H. leporispalustris and rabbits have been conducted in California (11,23–26). In addition to the initial report of Rickettsia sp. CA6269, a further 2 studies have reported SFG Rickettsia detections in H. leporispalustris; 1 of those studies described the isolation of a Rickettsia antigenically related to R. rickettsii (11,23,24). Although likely rare, the case-patients described in this study might have encountered questing H. leporispalustris infected with Rickettsia sp. CA6269. Alternatively, D. occidentalis, a tick vector that more frequently bites humans and parasitizes a wide variety of mammals including lagomorphs, the preferred host of adult H. leporispalustris, might have been the source of Rickettsia sp. CA6269 transmission (27,28).
As with R. rickettsii, Rickettsia sp. CA6269 is likely a rarely encountered pathogen in California (7). After the initial detection of Rickettsia sp. CA6269, retrospective testing of 8 SFG rickettsiosis patient samples revealed a second case from nearly 20 years earlier. Nucleic acids from both cases lacked a R. rickettsii–specific 23S rRNA SNP but were detected with the RRi6 assay, indicating cross-reactivity of the RRi6 assay with Rickettsia sp. CA6269. The RRi6 assay was designed as R. rickettsii–specific on the basis of comparative genome analyses and targets a gene encoding a hypothetical protein absent in other rickettsial genomes including Rickettsia 364D (13,14). A homologue to this target sequence is likely present within the Rickettsia sp. CA6269 genome, creating the potential for misidentification of Rickettsia sp. CA6269 as R. rickettsii when using the RRi6 assay. To address this issue, we developed a Rickettsia sp. CA6269–specific rPCR assay that provides excellent analytical specificity and sensitivity. With more extensive performance characterization, this assay should prove useful for diagnostic testing and environmental screening of potential vectors and reservoir hosts of Rickettsia sp. CA6269.
In summary, we have identified Rickettsia sp. CA6269 as a causative agent of severe RMSF-like illness in California and have described an assay for its detection. The application of this new test, both prospectively and retrospectively, could identify additional cases, thereby leading to a better understanding of both the clinical spectrum of disease caused by Rickettsia sp. CA6269 and the relative contribution of this pathogen to the increasing incidence of SFG rickettsioses in the United States. In addition, the new test will enable environmental studies to define the ecologic epidemiology of this emerging pathogen.
Dr. Probert is a research scientist with the Viral and Rickettsial Disease Laboratory of the California Department of Public Health. He is interested in the development of molecular diagnostic assays for the detection and genotyping of microbial pathogens.
Acknowledgments
We thank Chris Paddock and Joy Hecht for providing the Rickettsiaceae nucleic acids and thank Summer Adams, Alexa Quintana, Ricardo Berumen, Sabrina Gilliam, Brandon Stavig, Bianca Gonzaga, and Maria Salas for technical and medical records support. We would also like to thank Darvin Scott Smith for participating in the California Encephalitis Project and facilitating specimen submission. We also gratefully acknowledge Mary Joyce Pakingan and Erin Trent for tick extraction, Contra Costa Mosquito and Vector Control District for tick collection, and Alameda County Vector Control Services District for both tick collection and extraction efforts.
This work is funded in part by the Centers for Disease Control and Prevention Epidemiology and Laboratory Capacity for Infectious Diseases program (grant Number 5 NU50CK000539).
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views or opinions of the California Department of Public Health or the California Health and Human Services Agency.
References
- Bishop A, Borski J, Wang HH, Donaldson TG, Michalk A, Montgomery A, et al. Increasing incidence of spotted fever group rickettsioses in the United States, 2010–2018. Vector Borne Zoonotic Dis. 2022;22:491–7. DOIPubMedGoogle Scholar
- Blanton LS. The rickettsioses: a practical update. Infect Dis Clin North Am. 2019;33:213–29. DOIPubMedGoogle Scholar
- Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–96. DOIPubMedGoogle Scholar
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1–44. DOIPubMedGoogle Scholar
- Binder AM, Nichols Heitman K, Drexler NA. Diagnostic methods used to classify confirmed and probable cases of spotted fever rickettsioses—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2019;68:243–6. DOIPubMedGoogle Scholar
- California Department of Public Health. IDB yearly summaries of selected communicable diseases in California, 2013–2021 [cited 2023 Oct 20]. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/YearlySummSelectedGeneralCommDisinCA.aspx
- Kjemtrup AM, Padgett K, Paddock CD, Messenger S, Hacker JK, Feiszli T, et al. A forty-year review of Rocky Mountain spotted fever cases in California shows clinical and epidemiologic changes. PLoS Negl Trop Dis. 2022;16:
e0010738 . DOIPubMedGoogle Scholar - Paddock CD, Yoshimizu MH, Zambrano ML, Lane RS, Ryan BM, Espinosa A, et al. Rickettsia species isolated from Dermacentor occidentalis (Acari: Ixodidae) from California. J Med Entomol. 2018;55:1555–60. DOIPubMedGoogle Scholar
- Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, et al. The Eco-epidemiology of Pacific coast tick fever in California. PLoS Negl Trop Dis. 2016;10:
e0005020 . DOIPubMedGoogle Scholar - Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, et al. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin Infect Dis. 2010;50:541–8. DOIPubMedGoogle Scholar
- Eremeeva ME, Weiner LM, Zambrano ML, Dasch GA, Hu R, Vilcins I, et al. Detection and characterization of a novel spotted fever group Rickettsia genotype in Haemaphysalis leporispalustris from California, USA. Ticks Tick Borne Dis. 2018;9:814–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Spotted fever rickettsioses (including Rocky Mountain spotted fever) (SFR, including RMSF) 2020 case definition [cited 2023 Oct 20]. https://ndc.services.cdc.gov/case-definitions/spotted-fever-rickettsiosis-2020
- Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51:314–7. DOIPubMedGoogle Scholar
- Denison AM, Amin BD, Nicholson WL, Paddock CD. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis. 2014;59:635–42. DOIPubMedGoogle Scholar
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–65. DOIPubMedGoogle Scholar
- Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4:663–74. DOIPubMedGoogle Scholar
- Chung M, Munro JB, Tettelin H, Dunning Hotopp JC. Using core genome alignments to assign bacterial species. mSystems. 2018;3:e00236–18. DOIPubMedGoogle Scholar
- Diop A, El Karkouri K, Raoult D, Fournier PE. Genome sequence-based criteria for demarcation and definition of species in the genus Rickettsia. Int J Syst Evol Microbiol. 2020;70:1738–50. DOIPubMedGoogle Scholar
- Freitas LH, Faccini JL, Labruna MB. Experimental infection of the rabbit tick, Haemaphysalis leporispalustris, with the bacterium Rickettsia rickettsii, and comparative biology of infected and uninfected tick lineages. Exp Appl Acarol. 2009;47:321–45. DOIPubMedGoogle Scholar
- Kohls GM. Records and new synonymy of new world Haemaphysalis ticks, with descriptions of the nymph and larva of H. juxtakochi Cooley. J Parasitol. 1960;46:355–61. DOIPubMedGoogle Scholar
- Burgdorfer W. Ecology of tick vectors of American spotted fever. Bull World Health Organ. 1969;40:375–81.PubMedGoogle Scholar
- Nieto NC, Porter WT, Wachara JC, Lowrey TJ, Martin L, Motyka PJ, et al. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One. 2018;13:
e0199644 . DOIPubMedGoogle Scholar - Lane RS, Emmons RW, Dondero DV, Nelson BC. Ecology of tick-borne agents in California. I. Spotted fever group rickettsiae. Am J Trop Med Hyg. 1981;30:239–52. DOIPubMedGoogle Scholar
- Gurfield N, Grewal S, Doggett D, Ferran K, LaFreniere R. Detection of spotted fever group Rickettsia and Borrelia burgdorferi in San Diego County rabbit ticks. Proc Pap Annu Conf Mosq Vector Control Assoc Calif. 2011;79:25–6.
- Roth T, Lane RS, Foley J. A molecular survey for Francisella tularensis and Rickettsia spp. in Haemaphysalis leporispalustris (Acari: Ixodidae) in northern California. J Med Entomol. 2017;54:492–5.PubMedGoogle Scholar
- Schmitz KM, Foley JE, Kasten RW, Chomel BB, Larsen RS. Prevalence of vector-borne bacterial pathogens in riparian brush rabbits (Sylvilagus bachmani riparius) and their ticks. J Wildl Dis. 2014;50:369–73. DOIPubMedGoogle Scholar
- Eisen L. Tick species infesting humans in the United States. Ticks Tick Borne Dis. 2022;13:
102025 . DOIPubMedGoogle Scholar - Lane RS, Burgdorfer W. Spirochetes in mammals and ticks (Acari: Ixodidae) from a focus of Lyme borreliosis in California. J Wildl Dis. 1988;24:1–9. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: May 30, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Will Probert, California Department of Public Health, Viral and Rickettsial Disease Laboratory, 850 Marina Bay Pkwy, Richmond, CA, 94804, USA
Top