Volume 30, Number 7—July 2024
Research Letter
Serosurvey of Blood Donors to Assess West Nile Virus Exposure, South-Central Spain
Cite This Article
Citation for Media
Abstract
We analyzed West Nile Virus (WNV) exposure from 1,222 blood donors during 2017–2018 from an area of south-central Spain. Results revealed WNV seroprevalence of 0.08% (95% CI 0.004%–0.4%) in this population. Our findings underscore the need for continued surveillance and research to manage WNV infection in this region.
West Nile virus (WNV), a member of the family Flaviviridae, genus Orthoflavivirus, is classified within the Japanese encephalitis virus (JEV) serocomplex (1). It is the most widespread arbovirus globally, primarily because of the abundance and broad distribution of its main competent vector, mosquitoes belonging to the genus Culex (2). During the past 2 decades, WNV has led to epidemic outbreaks with a substantial proportion of severe cases in Europe, emerging as a considerable threat to public and animal health in these regions. Nonetheless, very limited information exists on seroprevalence in the general population, hindering a comprehensive understanding of the virus’ epidemiologic landscape.
In Spain, WNV is considered endemic because of conducive conditions for virus maintenance and circulation, including diverse bird reservoirs, geographic characteristics such as migratory bird routes, and specific climatic conditions. Since a notable outbreak reported in 2020, the virus has produced human cases annually (3), demonstrating the spread of the virus in the country (4). Therefore, vigilant surveillance in new risk areas is imperative to anticipate potential human health emergencies. Studies in vectors and animal hosts in south-central Spain have underscored the region’s potential as a hotspot zone (5–7). Within this area, the province of Ciudad Real, where no human WNV cases have been reported to date, serves as an ideal scenario for assessing circulation of the virus in the general population. We conducted a serosurvey in blood donors to investigate WNV exposure in the general population of this region in Spain, shedding light on the transmission dynamics of this emergent virus.
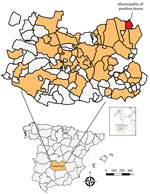
We conducted a retrospective cross-sectional study to analyze the seroprevalence of WNV in serum samples collected from blood donors at the Transfusion Center of the Hospital General Universitario de Ciudad Real (south-central Spain) (Figure) during 2017–2018 (Appendix). We selected and analyzed blood from 1,222 donors (Appendix Table 1). Sex and age data were not available for 129 (10.5%) donors. Of the 1,093 donors for whom information was available, 571 (52.2%) were men and 522 (47.8%) women. The age of the donors was categorized into 3 classes: <30 years (21.8% of samples), 30–50 years (34.8%), and >50 years (32.7%). Nineteen (1.6%) of the samples reacted positively to the IgG WNV ELISA. We administered an epidemiologic survey to the 19 ELISA-positive donors; 16 donors responded (Appendix Table 2).
We analyzed all ELISA-positive samples by using a virus neutralization test (VNT) (Appendix Table 2). Regarding WNV, ELISA reactivity was only confirmed by VNT in 1 donor who showed a titer of 1/256, which indicated a seroprevalence of 0.08% (95% CI 0.04%–0.4%) for WNV. This donor declared that he had not traveled outside of Spain and therefore did not receive any vaccine against yellow fever virus, tick-borne encephalitis virus, or Japanese encephalitis virus.
In Europe, no seroepidemiologic studies have been conducted since 2013; therefore, our study would provide valuable insights into the current status of WNV exposure. Our study encompasses a vast region of south-central Spain and marks initial identification of seropositivity in humans in this specific region of Spain, indicating a broad spread of the virus. In Spain, recent serosurveys are lacking; 2 studies were conducted in Catalonia in 2001 (0.2%) (8) and 2011 (0.12%) (9), and another was conducted in the province of Sevilla in 2006 (0.6%) (10). In the past 3 years, the regions of those studies have experienced large WNV outbreaks, similar to that which occurred in summer of 2020 (3) or the first description of clinical cases in Catalonia in 2022 and 2023 (4). This development suggests greater exposure to the virus than in the previous decade and highlights the need to carry out new serosurveys in the general population that enable collection of updated data.
The observed seroprevalence among blood donors from south-central Spain in our study suggests a low exposure (0.08%) to WNV in the general population within this spatiotemporal context. Of note, the number of WNV cases in Spain has been on the rise in recent years, being detected even in areas where previously no evidence of WNV circulation existed, suggesting that WNV has been expanding during recent years and that outbreaks can be expected in areas not currently considered endemic for WNV.
In our study, and in line with other studies (9), a high percentage (94.7%) of ELISA-positive WNV samples could not be confirmed as positive for specific antibodies. This finding highlights the need to perform additional neutralization tests against other flaviviruses in the serosurvey studies. The absence of an ELISA test with high sensitivity and, more crucially, specificity for WNV, limits the design of large-scale population serosurvey studies. Urgent efforts are required to address this limitation.
In conclusion, our study indicated seropositivity in the south-central region of Spain. In this way, reporting cases in Spain may be plausible even in areas not at high risk, highlighting the importance of ongoing surveillance and research to manage WNV infection in this region.
Dr. Frías is a postdoctoral researcher at the Animal Health and Zoonosis Research Group at the University of Cordoba and the Clinical Virology and Zoonoses Group at the Maimonides Biomedical Research Institute of Cordoba. His primary research interests are emerging zoonotic diseases.
Acknowledgments
We gratefully acknowledge Laura Ruiz Torres and Ismael Zafra Soto for their technical support in sample processing.
This work was supported by Secretaría General de Investigación, Desarrollo e Innovación en Salud (grant no. PI-0287-2019) for grants for the financing of Investigación, Desarrollo e Innovación Biomédica y en Ciencias de la Salud en Andalucía; the Ministerio de Sanidad (grant no. RD12/0017/0012) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional. A.R.J. is supported by a contract from the Spanish Junta de Andalucia (Nicolas Monardes program, grant no. C1-0001-2023). J.C.G. is supported by the CIBER Consorcio Centro de Investigación Biomédica en Red (grant no. CB21/13/00083), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea–NextGenerationEU.
References
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. DOIPubMedGoogle Scholar
- García San Miguel Rodríguez-Alarcón L, Fernández-Martínez B, Sierra Moros MJ, Vázquez A, Julián Pachés P, García Villacieros E, et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill. 2021;26:19. DOIPubMedGoogle Scholar
- Ministerio de Agricultura. Pesca y Alimentación. Update on the epidemiological situation of West Nile [cited 2024 May 14]. https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/informefno_tcm30-435293.pdf
- Durán-Martínez M. Distribución, Abundancia y Composición de la Comunidad de Dípteros Hematófagos Vectores de Enfermedades en Castilla-La Mancha: Riesgos para la Salud Pública y la Sanidad Animal. 2012 [cited 2024 Jan 25]. https://digital.csic.es/handle/10261/147173.
- García-Carrasco JM, Muñoz AR, Olivero J, Segura M, García-Bocanegra I, Real R. West Nile virus in the Iberian Peninsula: using equine cases to identify high-risk areas for humans. Euro Surveill. 2023;28:
2200844 . DOIPubMedGoogle Scholar - Casades-Martí L, Cuadrado-Matías R, Peralbo-Moreno A, Baz-Flores S, Fierro Y, Ruiz-Fons F. Insights into the spatiotemporal dynamics of West Nile virus transmission in emerging scenarios. One Health. 2023;16:
100557 . DOIPubMedGoogle Scholar - Bofill D, Domingo C, Cardeñosa N, Zaragoza J, de Ory F, Minguell S, et al. Human West Nile virus infection, Catalonia, Spain. Emerg Infect Dis. 2006;12:1163–4. DOIPubMedGoogle Scholar
- Piron M, Plasencia A, Fleta-Soriano E, Martinez A, Martinez JP, Torner N, et al. Low seroprevalence of West Nile virus in blood donors from Catalonia, Spain. Vector Borne Zoonotic Dis. 2015;15:782–4. DOIPubMedGoogle Scholar
- Bernabeu-Wittel M, Ruiz-Pérez M, del Toro MD, Aznar J, Muniain A, de Ory F, et al. West Nile virus past infections in the general population of Southern Spain. Enferm Infecc Microbiol Clin. 2007;25:561–5. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: June 18, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
|
|
|
|
|
|