Volume 31, Number 10—October 2025
Dispatch
Zoonotic Soil-Transmitted Helminth Infections among Humans, Gabon
Figure 2
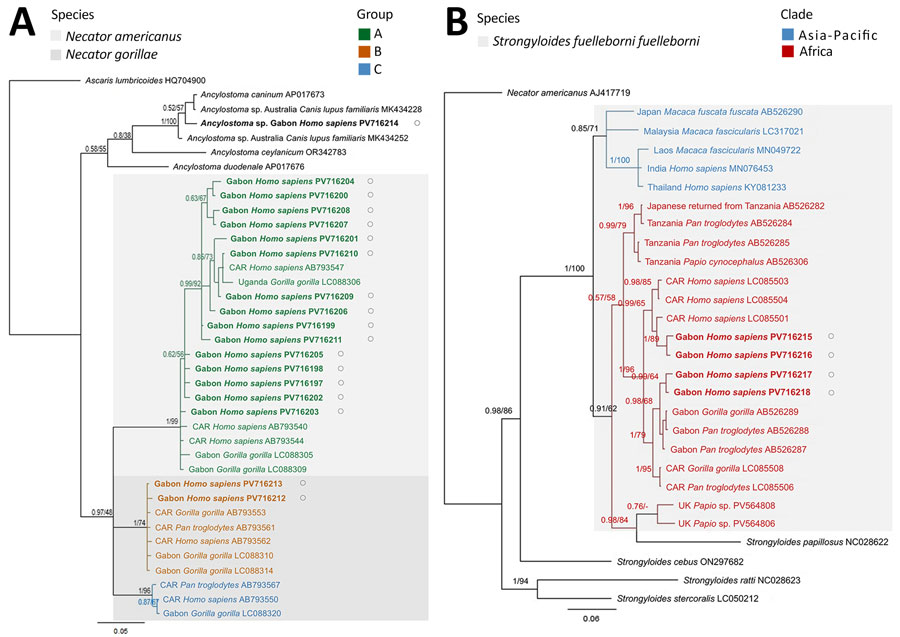
Figure 2. Maximum-likelihood phylogeny of zoonotic soil-transmitted helminths from human infections, Gabon. A) Necator spp. hookworms; color-coded groups are labeled per nomenclature by Hasegawa et al. (4). B) Strongyloides fuelleborni fuelleborni threadworms; color-coded groups represent geographic regions. Trees are based on cox1 sequences and were created by using MEGA 11 (https://www.megasoftware.net) and Bayesian inference using MrBayes (https://github.com/NBISweden/MrBayes). Bayesian posterior probability and maximum-likelihood bootstrap support percentages (1,000 bootstrap replicates) are indicated at the nodes. Bold font and black circles indicate sequences obtained in this study. Published sequences are annotated with the country of origin, host species, and GenBank accession numbers. Scale bars indicate nucleotide substitutions per site. CAR, Central African Republic
References
- Devaux CA, Mediannikov O, Medkour H, Raoult D. Infectious disease risk across the growing human-non human primate interface: a review of the evidence. Front Public Health. 2019;7:305. DOIPubMedGoogle Scholar
- Vancutsem C, Achard F, Pekel J-F, Vieilledent G, Carboni S, Simonetti D, et al. Long-term (1990–2019) monitoring of forest cover changes in the humid tropics. Sci Adv. 2021;7:eabe1603. DOIGoogle Scholar
- Kalousová B, Hasegawa H, Petrželková KJ, Sakamaki T, Kooriyma T, Modrý D. Adult hookworms (Necator spp.) collected from researchers working with wild western lowland gorillas. Parasit Vectors. 2016;9:75. DOIPubMedGoogle Scholar
- Hasegawa H, Modrý D, Kitagawa M, Shutt KA, Todd A, Kalousová B, et al. Humans and great apes cohabiting the forest ecosystem in central african republic harbour the same hookworms. PLoS Negl Trop Dis. 2014;8:
e2715 . DOIPubMedGoogle Scholar - Potters I, Micalessi I, Van Esbroeck M, Gils S, Theunissen C. A rare case of imported Strongyloides fuelleborni infection in a Belgian student. Clin Infect Pract. 2020;7–8:
100031 . DOIGoogle Scholar - Hasegawa H, Shigyo M, Yanai Y, McLennan MR, Fujita S, Makouloutou P, et al. Molecular features of hookworm larvae (Necator spp.) raised by coproculture from Ugandan chimpanzees and Gabonese gorillas and humans. Parasitol Int. 2017;66:12–5. DOIPubMedGoogle Scholar
- Young KH, Bullock SL, Melvin DM, Spruill CL. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J Clin Microbiol. 1979;10:852–3. DOIPubMedGoogle Scholar
- Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, Formenti F, et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Negl Trop Dis. 2019;13:
e0007609 . DOIPubMedGoogle Scholar - Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11:
e0005752 . DOIPubMedGoogle Scholar - Hasegawa H, Kalousova B, McLennan MR, Modry D, Profousova-Psenkova I, Shutt-Phillips KA, et al. Strongyloides infections of humans and great apes in Dzanga-Sangha Protected Areas, Central African Republic and in degraded forest fragments in Bulindi, Uganda. Parasitol Int. 2016;65(5 Pt A):367–70. DOIPubMedGoogle Scholar
- Richins T, Sapp SGH, Ketzis JK, Willingham AL, Mukaratirwa S, Qvarnstrom Y, et al. Genetic characterization of Strongyloides fuelleborni infecting free-roaming African vervets (Chlorocebus aethiops sabaeus) on the Caribbean island of St. Kitts. Int J Parasitol Parasites Wildl. 2023;20:153–61. DOIPubMedGoogle Scholar
- Pafčo B, Kreisinger J, Čížková D, Pšenková-Profousová I, Shutt-Phillips K, Todd A, et al. Genetic diversity of primate strongylid nematodes: Do sympatric nonhuman primates and humans share their strongylid worms? Mol Ecol. 2019;28:4786–97. DOIPubMedGoogle Scholar
- Ilík V, Kreisinger J, Modrý D, Schwarz EM, Tagg N, Mbohli D, et al. High diversity and sharing of strongylid nematodes in humans and great apes co-habiting an unprotected area in Cameroon. PLoS Negl Trop Dis. 2023;17:
e0011499 . DOIPubMedGoogle Scholar - Hira PR, Patel BG. Human strongyloidiasis due to the primate species Strongyloides fülleborni. Trop Geogr Med. 1980;32:23–9.PubMedGoogle Scholar
- de Ree V, Nath TC, Barua P, Harbecke D, Lee D, Rödelsperger C, et al. Genomic analysis of Strongyloides stercoralis and Strongyloides fuelleborni in Bangladesh. PLoS Negl Trop Dis. 2024;18:
e0012440 . DOIPubMedGoogle Scholar