Volume 31, Number 12—December 2025
Dispatch
Healthcare Worker Attitudes and Perceptions toward Ebola Vaccine, United States, 2024
Cite This Article
Citation for Media
Abstract
Our 2024 survey of eligible US healthcare workers found that 48% of unvaccinated healthcare workers are interested in receiving Ebola virus vaccine. The Advisory Committee on Immunization Practices recommended vaccination for healthcare workers at highest risk for occupational exposure to Zaire ebolavirus (Orthoebolavirus zairense). Addressing concerns identified by survey respondents might improve vaccine acceptance.
Orthoebolaviruses can be transmitted zoonotically or through exposure to bodily fluids from patients with Ebola disease (1). Orthoebolavirus zairense (Zaire ebolavirus) is the species most associated with outbreaks (2). During the 2014–2016 Zaire ebolavirus epidemic in West Africa, healthcare workers (HCWs) were infected and died from Ebola virus disease (EVD) at much higher rates than the general population (3). Four EVD cases were diagnosed in the United States, including 2 nurses involved in the care of an infected patient (4).
In 2019, the US Food and Drug Administration approved the Ebola vaccine ERVEBO (Merck, https://www.merck.com). The US Advisory Committee on Immunization Practices (ACIP) recommends the vaccine as a preexposure vaccination for persons at the highest risk of occupational exposure to Zaire ebolavirus (5,6). Eligible occupational risk groups include those responding to an outbreak, working as personnel at special pathogens treatment centers, or working as laboratorians handling specimens that might contain Zaire ebolavirus.
ERVEBO is highly effective in preventing disease caused by Zaire ebolavirus. The vaccine is a single-dose intramuscular injection that uses a live, attenuated recombinant vesicular stomatitis virus (VSV) to display the Ebola virus (strain Kikwit 1995) surface glycoprotein. An open-label, cluster-randomized ring vaccination trial done in Guinea and Sierra Leone estimated a vaccine efficacy of 100% (95% CI 79.3%–100.0%) (7). Subsequent observational studies also demonstrated vaccine effectiveness against Ebola virus transmission and death (8,9).
The real-world effectiveness of the vaccine in preventing EVD also depends on the willingness of HCWs to receive it. In this article, we describe the perceptions, attitudes, and desire to be vaccinated with ERVEBO among a sample of eligible HCWs from the United States.
We conducted a cross-sectional online anonymous survey (Appendix) through REDCap (10) during March–October 2024. We distributed the survey to HCWs eligible for ERVEBO vaccination on the basis of ACIP guidelines (5,6) at 3 Regional Emerging Special Pathogen Treatment Centers (RESPTCs): NYC Health + Hospitals/Bellevue (New York, NY, USA); University of Texas Medical Branch (Galveston, TX, USA); and Denver Health and Hospital Authority (Denver, CO, USA) (11). We selected those survey sites because they were planning to offer ERVEBO vaccine to eligible staff. The study was approved by the New York University Grossman School of Medicine Institutional Review Board (approval no. i23-00730). We conducted recruitment through email communications (68%) and in-person invitations during optional vaccine education sessions (32%). The cohort we labeled as “interested in receiving vaccine” was made up of respondents who indicated they were already vaccinated or would definitely or probably choose to be vaccinated. The cohort we labeled as “not interested in receiving vaccine or unsure” was made up of respondents who indicated they would definitely or probably not choose to be vaccinated or were unsure. We compared characteristics of the 2 cohorts by using a χ2 test and considered a p value <0.05 significant.
There were 66 respondents (37% response rate). The largest age group was 30–49 years of age (64%); 63.6% were female and 36.4% male (Table 1). Occupations represented included physicians (42%), nurses (27%), clinical laboratory professionals (24%), emergency medical technicians (5%), and research laboratory professionals (2%). The most represented hospital departments were critical care medicine (27%), hospital medicine (11%), infectious diseases (11%), and pediatric critical care (11%). More than half of participants (56%) had received some form of education on Ebola vaccines, most commonly through informational sheets or pamphlets (33%) or self-study of primary literature or public health guidelines (20%).
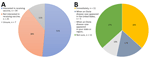
Thirty-four respondents (51%) were interested in receiving the vaccine, with a subset of respondents already vaccinated (n = 4) at the time of the survey (Figure, panel A). Among respondents interested and not already vaccinated, 27% would plan to get vaccinated when an EVD case appeared in the United States or their state or region (Figure, panel B).
Among those respondents unsure about or not interested in receiving the vaccine (n = 32), some reported they might be convinced to vaccinate if there were an EVD outbreak in the United States (44%); if they better understood the risks and benefits of vaccination (34%), vaccine safety (31%), or the risk of spreading VSV to others (28%); and if there were an Ebola virus vaccine using a different vaccine technology (19%). In this cohort, the most common reasons for not wanting to receive ERVEBO vaccination or being unsure of their decision included concerns about spreading VSV to others (44%), feeling that the risks of vaccine side effects are not acceptable (31%), not personally knowing enough about the vaccine to make a decision (31%), and not thinking there will be an EVD outbreak in the United States (28%).
Among all respondents, the most common concerns regarding side effects were the potential for serious side effects that lasted a long time or interfered with daily life (62%), the potential increased risk of spreading VSV to others (47%), and the potential increased risk for arthritis (36%). Many respondents expressed desire to learn more about ERVEBO (Table 2). The most selected educational topics of interest included the likelihood and nature of side effects from vaccination (67%), the likelihood and severity of spreading VSV to others (59%), and what their individual risk of getting EVD was (26%).
In 2022, ACIP expanded their occupational exposure to include laboratory workers within the Laboratory Response Network and staff at SPTCs. They conducted a knowledge, attitudes, and practices survey in October 2020, during an EVD outbreak in the Democratic Republic of the Congo (6). Among SPTCs, 54% of respondents reported willingness to be vaccinated. Vaccine willingness increased to 81% if they were given the choice of vaccination timing. Those responses are consistent with our findings that many HCWs interested in ERVEBO vaccination would prefer to wait until an EVD case is imported to the United States. However, the SPTC survey did not report on specific areas of interest for more education or what factors might influence currently uninterested HCWs to get vaccinated in the future.
Surveys conducted in the Democratic Republic of the Congo (12) and Uganda (13) indicated a strong interest among HCWs to receive an Ebola vaccine. However, those data might not be generalizable to HCWs in the United States because the risk for exposure to Ebola virus is lower. A survey in 2015 during the height of the 2014–2016 Ebola outbreak in West Africa found that only 34% of the US population were interested in receiving an Ebola vaccine (14). This survey did not specifically assess HCW interest, and it was performed before the US Food and Drug Administration licensure of ERVEBO.
During a period with no EVD outbreaks, 48% (30/62) of eligible unvaccinated HCWs surveyed at 3 US RESPTCs were interested in receiving the ERVEBO Ebola vaccine. Of those interested and not already vaccinated, 27% preferred to postpone vaccination until there is a case of EVD in the United States or their state or region.
One limitation of this study is that only 3 RESPTCs were surveyed, so their attitudes might not be representative of all US HCWs eligible for ERVEBO vaccination. Second, the sample size is too small to analyze for differences between subgroups of respondents. However, we did achieve a 37% survey response rate from a cohort of workers who are most likely to provide ongoing direct care to patients with EVD that require care in the United States.
Deployment of ERVEBO vaccine to eligible HCWs in the United States might be optimized by addressing respondent concerns. Those concerns include improving education on the risks and benefits of ERVEBO vaccination, vaccine safety, and risk of vaccine viral vector transmission.
Dr. Goswami is Medical Director for Travel Medicine at BronxCare Health System and a clinical assistant professor at the Icahn School of Medicine at Mount Sinai. Her clinical and research interests include emerging infectious diseases, biohazardous threats, and travel medicine.
Acknowledgment
A.L.P., R.M., M.F., C.L., and J.C. are members of their Regional Emerging Special Pathogen Treatment Center and the National Emerging Special Pathogen Treatment Center, which both receive funding from the Administration for Strategic Preparedness and Response.
References
- Jacob ST, Crozier I, Fischer WA II, Hewlett A, Kraft CS, Vega MA, et al. Ebola virus disease. Nat Rev Dis Primers. 2020;6:13. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Ebola (Ebola virus disease): outbreaks [cited 2025 Jun 23]. https://www.cdc.gov/ebola/outbreaks/index.html
- Evans DK, Goldstein M, Popova A. Health-care worker mortality and the legacy of the Ebola epidemic. Lancet Glob Health. 2015;3:e439–40. DOIPubMedGoogle Scholar
- Chevalier MS, Chung W, Smith J, Weil LM, Hughes SM, Joyner SN, et al.; Centers for Disease Control and Prevention (CDC). Ebola virus disease cluster in the United States—Dallas County, Texas, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1087–8.PubMedGoogle Scholar
- Choi MJ, Cossaboom CM, Whitesell AN, Dyal JW, Joyce A, Morgan RL, et al. Use of Ebola vaccine: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2021;70:1–12. DOIPubMedGoogle Scholar
- Malenfant JH, Joyce A, Choi MJ, Cossaboom CM, Whitesell AN, Harcourt BH, et al. Use of Ebola vaccine: expansion of recommendations of the advisory committee on immunization practices to include two additional populations—United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:290–2. DOIPubMedGoogle Scholar
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389:505–18. DOIPubMedGoogle Scholar
- Meakin S, Nsio J, Camacho A, Kitenge R, Coulborn RM, Gignoux E, et al.; Epicentre-MSF EVD Working Group. Effectiveness of rVSV-ZEBOV vaccination during the 2018-20 Ebola virus disease epidemic in the Democratic Republic of the Congo: a retrospective test-negative study. Lancet Infect Dis. 2024;24:1357–65. DOIPubMedGoogle Scholar
- Coulborn RM, Bastard M, Peyraud N, Gignoux E, Luquero F, Guai B, et al. Case fatality risk among individuals vaccinated with rVSVΔG-ZEBOV-GP: a retrospective cohort analysis of patients with confirmed Ebola virus disease in the Democratic Republic of the Congo. Lancet Infect Dis. 2024;24:602–10. DOIPubMedGoogle Scholar
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. DOIPubMedGoogle Scholar
- National Emerging Special Pathogens Training and Education Center. Partners and regional contacts [cited 2025 Jun 23]. https://netec.org/about-netec/partners-regional-contacts
- Doshi RH, Garbern SC, Kulkarni S, Perera SM, Fleming MK, Muhayangabo RF, et al. Ebola vaccine uptake and attitudes among healthcare workers in North Kivu, Democratic Republic of the Congo, 2021. Front Public Health. 2023;11:
1080700 . DOIPubMedGoogle Scholar - Waltenburg MA, Kainulainen MH, Whitesell A, Nyakarahuka L, Baluku J, Kyondo J, et al. Knowledge, attitudes, and practices and long-term immune response after rVSVΔG-ZEBOV-GP Ebola vaccination in healthcare workers in high-risk districts in Uganda. Vaccine. 2024;42:
126031 . DOIPubMedGoogle Scholar - Painter JE, DiClemente RJ, von Fricken ME. Interest in an Ebola vaccine among a U.S. national sample during the height of the 2014-2016 Ebola outbreak in West Africa. Vaccine. 2017;35:508–12. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: December 24, 2025
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Justin Chan, NYC Health + Hospitals/Bellevue, 462 1st Ave, H-building 6W57, New York, NY 10016, USA
Top