Volume 31, Number 2—February 2025
Research
Contribution of Limited Molecular Testing to Low Ehrlichiosis Diagnosis in High Incidence Area, North Carolina, USA
Abstract
Indirect immunofluorescence antibody assays have been the primary method for laboratory diagnosis of ehrlichiosis. Detection of Ehrlichia spp. DNA by using PCR is now widely available through commercial laboratories. To prepare for Ehrlichia spp. PCR introduction, we assessed ehrlichiosis testing practices, quantified the proportion of samples eligible for PCR testing, and estimated the potential effect of implementing PCR at the University of North Carolina health system in North Carolina, USA, which is in an area with a high-incidence of ehrlichiosis. We found <1% of patient samples underwent PCR testing, even though rates of serodiagnostic algorithm completion (testing of acute and convalescent samples) were low (18.4%). Our findings show a need to educate providers on diagnostic and treatment guidelines for ehrlichiosis and raise awareness of the availability and advantage of PCR testing.
Since 2000, the number of ehrlichiosis cases in the United States reported to the Centers for Disease Control and Prevention (CDC) has increased >10-fold (1). Yet, ehrlichiosis is frequently misdiagnosed and underreported because of its relatively nonspecific clinical manifestations (e.g., fever, malaise, headache, myalgia) (2). Whereas associated laboratory abnormalities such as thrombocytopenia, hepatic transaminase elevation, and leukopenia may provide further diagnostic clues, similar abnormalities can be seen in other infections. The potential consequences of misdiagnosis are substantial because Ehrlichia spp. infection can lead to severe disease. For example, 57% of ehrlichiosis cases reported to CDC during 2008–2012 (1,584 cases) resulted in hospitalization (3). Delayed antimicrobial drug treatment is strongly associated with clinical deterioration, characterized by organ failure and death, particularly in children and older adults (4,5).
Historically, paired acute and convalescent serum samples tested by using an indirect immunofluorescence antibody (IFA) assay that detects IgG against E. chaffeensis antigens have been the primary method of laboratory diagnosis (6). However, the serodiagnosis of ehrlichiosis is error-prone. IgG may not be detectable early in the course of infection, when most patients seek care (7). In addition, a positive acute serologic result may not indicate current infection but rather a prior infection, which may occur in ≈10% of the population in endemic areas (8,9). Because of the possibility of prior infection, a single acute serologic result cannot be used to confirm a diagnosis, and clinicians must make treatment decisions on the basis of a thorough clinical evaluation, considering a patient’s history, symptoms, and laboratory test results. Providers often wait for the results of the initial IFA assay before beginning antimicrobial drug treatment (10), which can lead to disease progression. A second convalescent sample taken 2–10 weeks after the initial acute serologic result is required to confirm the diagnosis of ehrlichiosis and for epidemiologic surveillance data (11).
Few patients complete both acute and convalescent testing. For example, in North Carolina, of 105 cases reported in 2020, only 14 (13.3%) were classified as confirmed because of the lack of a paired convalescent result (12). Even within a large academic medical center, only 1 in 4 patients tested for ehrlichiosis completed a convalescent test (13). Multiple factors may explain the low obtainment of paired serologic results, including the resolution of clinical symptoms because of treatment or self-limited disease, evidence of another cause of illness, or lack of clinician knowledge of testing algorithms.
Molecular approaches that use PCR can detect Ehrlichia spp. DNA with high sensitivity and specificity in acute whole blood samples, thereby eliminating the need for convalescent specimens to confirm the diagnosis (14). Although treatment for ehrlichiosis must be initiated presumptively, positive Ehrlichia PCR results can provide confirmatory evidence of the diagnosis more quickly than paired acute and convalescent samples. Molecular assays can also differentiate between infecting species such as E. chaffeensis, E. ewingii, and E. muris eauclairensis, which can advance our understanding of distinct syndromes associated with each species. However, PCR does have limitations, including increased cost (≈5× that of paired acute and convalescent IFAs at our institution, the University of North Carolina [UNC; Chapel Hill, NC, USA]), uncertainty in the ability to detect Ehrlichia sp. DNA after antimicrobial drugs are administered, and the lack of Food and Drug Administration–approved assays.
In preparation for the implementation of a laboratory-developed Ehrlichia PCR, we assessed current diagnostic testing practices, quantified the proportion of serologically tested patients that would be eligible for PCR testing, and estimated the potential effect of PCR on the diagnosis and management of ehrlichiosis within the UNC Health System. To accomplish this goal, we conducted a retrospective chart review of all patients tested for Ehrlichia over a 12-month period. We hypothesized that serologic testing was poorly targeted, and obtainment of paired specimens (acute and convalescent) would be infrequent, whereas Ehrlichia PCR, previously available only through a commercial reference laboratory, was underused.
Data Sources
We obtained diagnostic test results from patients tested for ehrlichiosis as ordered through Epic, the electronic medical record (EMR) system for UNC Health. UNC Health is the largest academic health system in North Carolina, comprising 14 hospitals and ≈500 clinics located throughout the state (15). In 2018, UNC Health reported 3.5 million clinical visits, which included nearly 500,000 emergency department visits. Patients were tested for ehrlichiosis by using IFA (Biocell Diagnostics, Inc, http://biocelldx.com) through UNC’s McLendon Clinical Laboratories or by using PCR as a referral or send out test to Mayo Clinical Laboratories (Rochester, MN, USA), which was the institutional provider for Ehrlichia PCR testing during March 24, 2022–April 14, 2023 (16). Demographic, laboratory, and clinical data associated with each test order were entered into an electronic database (17).
Statistical Analysis
We stratified patients on the basis of 3 criteria: test appropriateness, PCR eligibility, and epidemiologic case classification. We first reviewed symptoms documented in the EMR to identify patients who were appropriately tested, defined as those who met the 2007 Council of State and Territorial Epidemiologists (CSTE) case definition for ehrlichiosis, which was in place at the time the samples were tested (18). Although the 2007 CSTE case definition was not intended to determine when to use diagnostics, it offers clear criteria for clinical assessment. Clinical evidence was defined as a subjectively reported or objectively measured fever (temperature >38°C) as documented in the EMR and >1 of the following symptoms: headache, myalgia, anemia (hemoglobin <13.5 g/dL for men, <12.0 g/dL for women), thrombocytopenia (platelets <150/µL), hepatic transaminase elevation (aspartate aminotransferase >33 U/L or alanine aminotransferase >36 U/L), or leukopenia (leukocyte count <4,000 cells/µL) (18). We classified patients as eligible for PCR testing if they were prescribed tetracycline antimicrobial drugs the same day as or after initial specimen collection because antimicrobial drugs can remove Ehrlichia DNA from patient blood and decrease the sensitivity of PCR testing (8). By using the CSTE case classifications, we determined the proportion of cases classified as confirmed, probable, or suspect. A confirmed case demonstrated a 4-fold or greater increase between acute and convalescent IgG titers and consistent clinical evidence of ehrlichiosis. A positive Ehrlichia PCR test with consistent clinical evidence was also considered a confirmed case. A probable case did not demonstrate a 4-fold increase but had >1 positive serologic specimen along with clinical evidence of ehrlichiosis. The threshold for a positive serologic test was a 1:64 IgG titer, selected to align with the cutoff used by the CDC to meet laboratory supportive evidence for ehrlichiosis (11). A case was classified as suspect if the patient had a positive laboratory test but no clinical information was available to determine if they had relevant symptoms. The number of cases in each category was summarized and shown with relevant proportions.
Ethical Review
The study was approved by the institutional review board of the University of North Carolina at Chapel Hill (institutional review board no. 21-0356). Because this is a limited dataset under Code of Federal Regulations 45, part 164.514(e), written informed consent or waiver of authorization was not required.
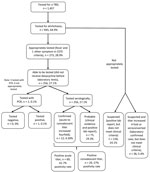
A total of 945 patient samples were tested for ehrlichiosis during the ≈12-month period observed, 5 of which underwent Ehrlichia PCR testing. Among all patients tested, the most frequently recorded symptoms at the time of testing were myalgia (33.9%, n = 320) and headache (31.3%, n = 296) (Table 1). Of those, only 273 (28.9%) were classified as appropriately tested; the most common reasons for exclusion were the absence of documented fever in the EMR (97.9%, n = 658), patient complaint including only 1 symptom (15.8%, n = 106), a lack of any symptoms, or failure to document any symptoms in the EMR (10.4%, n = 70), or a combination. Of the patients who underwent PCR testing, 4 of 5 were not classified as appropriately tested because of the absence of fever. Of note, among the patients excluded, 30.5% reported a tick bite in the 2 weeks before the visit. Most of the appropriately tested patients (93.8%, n = 256) had not received doxycycline at the time of sample collection and therefore would have been eligible for a PCR test (Figure). However, only 1 patient meeting clinical criteria (0.4%) underwent PCR, whereas the remaining had serologic testing performed.
Among the 256 patients who were eligible for PCR, 69 (27.0%) had an acute titer result of >1:64. Only 47 (18.4%) patients completed convalescent testing. Among those who underwent convalescent testing, 6 (12.8%) demonstrated seroconversion and 12 (17.4%) who were positive at acute testing showed a 4-fold or greater titer increase. In addition, 5 (10.6%) reverted from a positive to a negative titer (Table 2). Of note, 106 (38.8%) of 256 patients had a negative acute Ehrlichia IgG titer and were started on antimicrobial drugs but did not return for convalescent blood testing. Similarly, 58 (22.7%) of 256 patients had a negative acute titer and neither received antimicrobial drugs nor returned for a convalescent test. Of the 47 patients who completed acute and convalescent Ehrlichia IgG testing, the median number of days between tests was 22 (interquartile range 17–32).
Of the 5 patients who had whole blood samples collected and tested by PCR, 1 test was positive. Attending physicians in the emergency department were responsible for ordering 3 of 5 PCR tests. In all 5 cases, serologic testing for Ehrlichia was also conducted. Three of the 5 had an acute but not a convalescent Ehrlichia IgG test, whereas 4 of 5 were classified as not appropriately tested because no fever was reported in the EMR (all had >1 other symptom in the CSTE criteria). However, the single positive PCR sample was from a patient whose clinical manifestations included a fever and several other consistent symptoms. The send-out PCR test took an average of 6.2 days to receive a result from the reference laboratory.
Overall, 12 (4.7%) of 256 patients who were eligible for PCR were classified as confirmed ehrlichiosis cases (11 by serology and 1 by PCR and serology); and 75 (29.3%) were classified as probable cases. However, on further review of cases that we defined as not appropriately tested, 228 (33.9%) of 673 patients also had 1 positive Ehrlichia spp. test, including 193 (28.7%) who had a positive acute IgG test and 90 (13.4%) who had a convalescent IgG test. Among those who returned for a convalescent test, 35 patients showed seroconversion. Of probable cases, 7 exhibited a 4-fold IgG increase from acute to convalescent titers, raising the total number of laboratory-confirmed cases to 48.
Our study of routine diagnostic testing practices demonstrated that most patients suspected of having ehrlichiosis did not undergo PCR testing when it was available only as a send-out test, despite the multiple advantages of this testing method. Instead, providers continued to rely on serologic testing, even though rates of diagnostic algorithm completion were low (18.4%). Because the lone star tick (which does not transmit Rickettsia rickettsii, the causative bacteria of Rocky Mountain spotted fever) is the primary vector in the state (19), it is very likely that ehrlichiosis, along with R. parkeri, accounts for most symptomatic, tickborne illness episodes (2). The limited understanding of the diagnosis and management of ehrlichiosis and the underuse of Ehrlichia PCR as a confirmatory assay is particularly concerning. Those issues underscore the need to educate providers at all levels of training and across specialties on the diagnosis and management of ehrlichiosis. In addition, improved uptake of PCR can increase knowledge of the true rate of ehrlichiosis cases in the United States, specifically through a higher rate of confirmation and additional information regarding infecting species.
Several factors may explain the underuse of PCR testing. First, few health facilities have the capability or resources to perform an Ehrlichia PCR test in their own laboratories. At the time of this study, Ehrlichia PCR was only available at UNC Health as a referral test, with samples sent to Mayo Clinical Laboratories. Many providers may not have been aware that an Ehrlichia PCR test was available because it did not appear in the standard order menus. Instead, when ordering an Ehrlichia PCR, providers were required to order a generic referral test, which then launched a separate screen where the requested test can be entered as a free text. Ordering the test this way requires knowledge of the Ehrlichia PCR, the required sample type, and the receiving laboratory. In contrast, serologic testing, which is performed in-house, can be ordered by a simple search term lookup. This difference in processes might have affected ordering patterns, especially among nonspecialists who are not familiar with the test options. In addition, send-out PCR results still took nearly a week to return to the ordering provider. This timeline might have been insufficient for providers to perceive a benefit to guide treatment decisions, especially when other infections were on the differential diagnosis. Therefore, for optimal implementation, testing likely needs to be performed in-house, and the PCR order should be more prominent and easier to locate within routine test menus.
Whereas empirical antimicrobial drug treatment is recommended when there is a reasonable pretest probability of ehrlichiosis, the relatively fast turnaround of PCR, at least compared with paired serologic testing, offers a timely and objective result to confirm the diagnosis, thereby providing valuable information to both the patient and provider regarding the cause of illness. In our cohort, the number of patients for whom testing was ordered but were not empirically treated with doxycycline is concerning. Because the providers believed there was sufficient evidence to order an ehrlichiosis test, they also should have provided empirical treatment to patients. Without a convalescent blood test, an acute titer result cannot be used to either confirm or rule out ehrlichiosis. Positive PCR results can offer timelier confirmation of ehrlichiosis confirmation and subsequently ensure an appropriate treatment plan. Whereas treatment is ideally prescribed with clinical suspicion, findings show that this is not always the case, and a positive PCR may prompt more immediate action if otherwise not taken. Moreover, because convalescent testing has a much higher positivity rate than acute testing (61.7% vs. 27.0%) but most patients who receive a negative acute IgG titer fail to return for a convalescent titer, there are likely patients with ehrlichiosis who go undetected (2). Differing titer values in more than half of paired acute and convalescent samples indicate the convalescent test is clearly needed to interpret serologic results accurately, consistent with current guidelines (6). The relatively large proportion of paired titers of the same value implies prior infection and reinforces the importance of paired serologic specimens in areas where baseline seroprevalence is high.
Surprisingly, many patients tested for ehrlichiosis were considered not appropriately tested according to the 2007 CSTE criteria, primarily because of the absence of a subjectively reported fever as documented in the medical record. Yet, the number of laboratory-positive cases and seroconversion rates among patients without clinical evidence was notably higher than that for patients with clinical evidence (36 vs. 12). Those findings suggest that fever is not always a symptom associated with ehrlichiosis. It is also possible that fever is underrecognized because of antipyretic drugs used for headaches and pain control. Largely because of a lack of speciation with serodiagnostic testing, it is possible that infections with non–E. chaffeensis species, such as E. ewingii and E. muris eaclairensis, may exhibit different clinical manifestations than E. chaffeensis, which historically is the prototypical pathogen. Previous studies have identified E. ewingii in ticks across North Carolina, frequently in higher prevalence than E. chaffeensis, which has also been observed in other states (19,20). Those observations drove changes to the CSTE case definition, which removed fever as a required symptom.
Our study strengths include conduct in an area with high incidence of ehrlichiosis and the use of a robust electronic database. The first limitation of the study is its retrospective nature and that it relied on routine clinical records to determine clinical symptoms associated with manifestation. Second, unknown data, such as the presence of fever not being entered into a patient’s chart, might have resulted in the misclassification of patients. Third, the occurrence of and reliance on single serologic tests has major drawbacks, as described in this article. Fourth, there is potential uncertainty about the true disease state of the patients described in this study. Fifth, the study took place during the COVID-19 pandemic, when care-seeking patterns and diagnostic testing algorithms were disrupted. Finally, we do not have long-term outcomes to assess if participants experienced adverse clinical outcomes because of delayed or lack of antimicrobial drug administration resulting from incomplete testing.
In conclusion, our investigation revealed major underuse of molecular testing for ehrlichiosis in a disease-endemic area, despite the well-established issues associated with serologic testing. Our findings highlight the potential gains that can be made with increased uptake through both provider education and implementation of local testing. Molecular testing could provide information on infecting species, which could help clarify the clinical spectrum, epidemiology, and geographic distribution of different Ehrlichia species, and ultimately improve surveillance for this emerging disease and more accurately identify patients at risk of infection.
Ms. Siegler is an undergraduate student at the University of North Carolina at Chapel Hill studying neuroscience and political science. She is a research assistant at the vectorborne epidemiology, ecology, and response hub investigating the epidemiology of tickborne diseases.
Acknowledgments
We wish to thank the regulatory team at the University of North Carolina Institute of Global Health and Infectious Diseases, as well as the data management team at the Carolina Data Warehouse for their efforts.
Deidentified individual data from this study will be shared beginning 9–36 months following publication, provided the investigator who proposes to use the data has approval from an Institutional Review Board, Independent Ethics Committee, or Research Ethics Board, as applicable, and executes a data use or sharing agreement with the University of North Carolina.
Funding for this study was provided in part by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research (to R.M.B). This study was partially supported by the North Carolina Translational and Clinical Sciences Institute, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant award no. UL1TR002489).
Author contributions: study conception and design, R.M.B. and A.S.; funding, R.M.B.; implementation, A.S., L.T.U., and E.W.; data analysis, A.S. and R.M.B.; first draft, A.S. and R.M.B.; and manuscript revisions, all authors.
References
- Centers for Disease Control and Prevention. Ehrlichiosis epidemiology and statistics [cited 2023 May 13]. https://www.cdc.gov/ehrlichiosis/stats
- Boyce RM, Sanfilippo AM, Boulos JM, Cleinmark M, Schmitz J, Meshnick S. Ehrlichia Infections, North Carolina, USA, 2016. Emerg Infect Dis. 2018;24:2087–90. DOIPubMedGoogle Scholar
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:52–60. DOIPubMedGoogle Scholar
- Kuriakose K, Pettit AC, Schmitz J, Moncayo A, Bloch KC. Assessment of risk factors and outcomes of severe ehrlichiosis infection. JAMA Netw Open. 2020;3:
e2025577 . DOIPubMedGoogle Scholar - Hamburg BJ, Storch GA, Micek ST, Kollef MH. The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore). 2008;87:53–60. DOIPubMedGoogle Scholar
- Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1–44. DOIPubMedGoogle Scholar
- Bakken JS, Haller I, Riddell D, Walls JJ, Dumler JS. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin Infect Dis. 2002;34:22–7. DOIPubMedGoogle Scholar
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl 1):S45–51. DOIPubMedGoogle Scholar
- Zychowski DL, Alvarez C, Abernathy H, Giandomenico D, Choudhary SK, Vorobiov JM, et al. Tick-borne disease infections and chronic musculoskeletal pain. JAMA Netw Open. 2024;7:
e2351418 . DOIPubMedGoogle Scholar - Mokashi NV, Brown Marusiak A, Giandomenico D, Cleinmark M, Schmitz JL, Boyce RM. Does paging clinicians about tick-borne disease serological results impact clinical care? A retrospective analysis of 70 cases in North Carolina. Am J Trop Med Hyg. 2024;110:815–8. DOIPubMedGoogle Scholar
- Council of State and Territorial Epidemiologists. Update to public health reporting and national notification for ehrlichiosis. Vol. 23-ID-04. 2023 [cited 2024 Feb 2]. https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/ps_2023/23-ID-04_Ehrlichiosis.pdf
- North Carolina Division of Public Health. Ehrlichiosis surveillance from 2016–2021 [cited 2023 Jan 10]. https://epi.dph.ncdhhs.gov/cd/ehrlichiosis/EhrlichiosisSurveillanceSummary2021.pdf
- Brown Marusiak A, Hollingsworth BD, Abernathy H, Alejo A, Arahirwa V, Mansour O, et al. Patterns testing for tick-borne diseases and implications for surveillance in the southeastern US. JAMA Netw Open. 2022;5:
e2212334 . DOIPubMedGoogle Scholar - Eshoo MW, Crowder CD, Li H, Matthews HE, Meng S, Sefers SE, et al. Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol. 2010;48:472–8. DOIPubMedGoogle Scholar
- The University of North Carolina Health System. 2021 Annual report [cited 2023 Dec 20]. https://www.unchealthcare.org/app/files/public/9676dff4-3a41-42ce-b177-1a25647a0c62/pdf-UNC_Health_2021_Annual_Report.pdf
- Mayo Foundation for Medical Education and Research. TEST ID: EPCRB—Ehrlichia/Anaplasma, molecular detection, PCR, blood [cited 2023 Dec 21]. https://www.mayocliniclabs.com/test-catalog/Overview/618301#Performance
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. DOIPubMedGoogle Scholar
- Council of State and Territorial Epidemiologists. Revision of the national surveillance case definition for ehrlichiosis (Ehrlichiosis/Anaplasmosis). Vol. 07-ID-03. 2007 [cited 2024 Feb 21]. https://cdn.ymaws.com/www.cste.org/resource/resmgr/PS/07-ID-03.pdf
- Lee S, Kakumanu ML, Ponnusamy L, Vaughn M, Funkhouser S, Thornton H, et al. Prevalence of Rickettsiales in ticks removed from the skin of outdoor workers in North Carolina. Parasit Vectors. 2014;7:607. DOIPubMedGoogle Scholar
- Wolf L, McPherson T, Harrison B, Engber B, Anderson A, Whitt P. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J Clin Microbiol. 2000;38:2795. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: January 16, 2025
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ross M. Boyce, University of North Carolina at Chapel Hill, 123 W Franklin St, Ste 2151, Chapel Hill, NC 27516, USA
Top