Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 3—March 2025
Research Letter
Evaluation of High-Dose Isoniazid in Multidrug-Resistant Tuberculosis Treatment
Suggested citation for this article
Abstract
High-dose isoniazid is recommended to treat multidrug-resistant tuberculosis (MDR TB). Among 958 MDR TB isolates identified in France during 2008–2022, 93.1% exhibited high-level isoniazid resistance, and molecular testing showed limited diagnostic accuracy in predicting resistance. Clinicians should reconsider using high-dose isoniazid in MDR TB treatment because of suboptimal effect and toxicity concerns.
Despite the turning point represented by the 2022 update in World Health Organization guidelines (1), optimal treatment for multidrug-resistant tuberculosis (MDR TB), defined by isoniazid and rifampin resistance, remains a global challenge. The 6-month, all-oral regimen combining bedaquiline, pretomanid, linezolid, and moxifloxacin represents a breakthrough in MDR TB treatment; however, its adoption is limited by cost and access issues. Alternative treatments include the 9-month, all-oral short-course regimen (SCR) and the 18-month, individualized conventional regimen. The SCR is recommended for patients with MDR TB without fluoroquinolone resistance and includes high-dose isoniazid of 10–15 mg/kg. The conventional regimen may also incorporate high-dose isoniazid if there is no confirmation of high-level isoniazid resistance (1). The effectiveness of high-dose isoniazid relies on the absence of mutations known to confer phenotypic high-level isoniazid resistance, notably mutations in the katG gene (2). The high prevalence of such mutations among MDR TB isolates makes the role of high-dose isoniazid in MDR TB regimens questionable (3). We quantified the prevalence of high-level isoniazid resistance among MDR TB isolates, particularly isolates from patients eligible for SCR, and evaluated the diagnostic accuracy of molecular testing for predicting high-level isoniazid resistance.
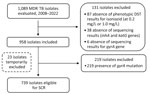
We used data from the comprehensive French national network of the National Reference Centre for Mycobacteria (Paris, France) to perform a retrospective, observational cohort study. We included the isolate obtained at diagnosis from each patient with confirmed pulmonary or extrapulmonary MDR TB identified in France during 2008–2022. We excluded isolates lacking phenotypic drug susceptibility testing (DST) results for isoniazid (0.2 and 1.0 µg/mL) or genotypic data for inhA, katG, and gyrA. For phenotypic DST, solid and liquid cultures were used interchangeably. Testing of solid cultures used the proportion method on Löwenstein-Jensen media, whereas testing of liquid cultures used mycobacteria growth indicator tubes containing Middlebrook 7H9 broth (BD Difco, https://www.bd.com). We defined resistance levels as low-level (0.2 µg/mL) or high-level (1.0 µg/mL) (4). Genotypic DST used GenoType MTBDRplus and MTBDRsl assays (Bruker, https://www.bruker.com) and Sanger or targeted next-generation sequencing (Deeplex Myc-TB; Genoscreen, https://www.genoscreen.fr) when line-probe assay results were missing or uninterpretable. We interpreted results according to the World Health Organization catalog of resistance-associated genetic variants (5). We defined eligibility for SCR as the absence of gyrA mutations.
We assessed the diagnostic accuracy of katG mutations (with or without mutation in inhA) in predicting high-level isoniazid resistance by using phenotypic DST. In the DST calculation, strains without mutations were excluded because they could not be classified as low-level resistance (inhA mutation) or high-level resistance (katG with or without inhA mutation) in phenotypic-genotypic comparisons.
Descriptive statistics included frequency analyses for categorical variables and median and interquartile range for quantitative variables. We calculated 95% CIs for proportions. We used Stata 15.2 (StataCorp LLC, https://www.stata.com) for analyses and considered p<0.05 statistically significant. Ethical approval was granted by the ethics review board of the Bligny Hospital, Briis-sous-Forges, France (study design approved by Conseil de Réflexion Ethique on June 27, 2023).
Among 1,089 MDR TB isolates, 958 were included in the study (Figure). Of those isolates, 892 (93.1%, 95% CI 91.5–94.7) exhibited high-level isoniazid resistance (Table). Mutations in katG were found in 837 (87.4%) isolates and inhA mutations in 259 (27.0%) isolates. Of note, 828 (98.9%) of the 837 isolates with katG mutations showed high-level isoniazid resistance. In addition, we detected high-level isoniazid resistance in 51.0% of isolates with inhA mutations and without katG mutations. Of the 739 isolates from patients eligible for the SCR (Figure), 677 (91.6%) had high-level isoniazid resistance. The diagnostic accuracy of genotypic testing for predicting high-level isoniazid resistance compared with phenotypic DST was as follows: sensitivity 93.3% (95% CI 91.6–94.9), specificity 86.4% (95% CI 84.2–88.6), positive predictive value 99.0% (95% CI 98.4–99.7), and negative predictive value 46.4% (95% CI 43.2–49.6). Those accuracy metrics were comparable among isolates from patients eligible for SCR (Table).
Our findings indicated that high-dose isoniazid is unlikely to be effective for most patients using the MDR TB regimen because of the high prevalence of high-level isoniazid resistance, including those from patients eligible for SCR. The high frequency of observed katG mutations aligns with previous studies; most katG mutant isolates exhibited high-level isoniazid resistance (3). Furthermore, the absence of katG mutations alone does not reliably exclude high-level isoniazid resistance because more than half of strains in our study with inhA mutations displayed isoniazid MICs >1.0 mg/L. Although high-dose isoniazid was previously considered effective against inhA mutant isolates (6), more recent research reported MICs >1.0 mg/L in those strains (7,8), which limits the utility of genotypic testing in predicting low-level isoniazid resistance. Although high-dose isoniazid may still be appropriate in specific cases, the associated toxicity risks suggest that its inclusion in MDR TB regimens may not be warranted (9,10).
In summary, high-dose isoniazid offers limited benefit for most patients using the MDR TB regimen because of widespread high-level isoniazid resistance. Clinicians should optimize existing regimens, replace high-dose isoniazid with safer, more effective alternatives, and promote global access to new treatments.
Dr. Gerussi is an infectious diseases specialist at the Infectious Diseases Department of Trieste University Hospital, Trieste, Italy. Her research interests include tubercular infections and antibiotic drug use.
Acknowledgments
The authors thank the technical staff of the National Reference Centre for Mycobacteria (Paris, France) for their invaluable and meticulous daily contributions. V. Gerussi thanks Maria Merelli for her support and Carlo Tascini for providing the opportunity to conduct research at the National Reference Centre for Mycobacteria.
V.G., A.A., N.V., and L.G. are members of European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections.
References
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment, 2022 update. Geneva: The Organization; 2022.
- Gausi K, Ignatius EH, De Jager V, Upton C, Kim S, McKhann A, et al. High-dose isoniazid lacks EARLY bactericidal activity against isoniazid-resistant tuberculosis mediated by katG mutations: a randomized, phase 2 clinical trial. Am J Respir Crit Care Med. 2024;210:343–51. DOIPubMedGoogle Scholar
- Chesov D, Ciobanu N, Lange C, Heyckendorf J, Crudu V. High-dose isoniazid in the shorter-course multidrug-resistant tuberculosis regimen in the Republic of Moldova. Eur Respir J. 2017;50:
1701340 . DOIPubMedGoogle Scholar - World Health Organization. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). Geneva: The Organization; 2021.
- World Health Organization. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance, 2nd ed. Geneva: The Organization; 2023.
- Dooley KE, Miyahara S, von Groote-Bidlingmaier F, Sun X, Hafner R, Rosenkranz SL, et al.; A5312 Study Team. Early bactericidal activity of different isoniazid doses for drug-resistant TB (INHindsight): a randomized open-label clinical trial. Am J Respir Crit Care Med. 2020;201:1416–24. DOIPubMedGoogle Scholar
- Lale Ngema S, Dookie N, Perumal R, Nandlal L, Naicker N, Peter Letsoalo M, et al. Isoniazid resistance-conferring mutations are associated with highly variable phenotypic resistance. J Clin Tuberc Other Mycobact Dis. 2023;33:
100387 . DOIPubMedGoogle Scholar - Ghodousi A, Tagliani E, Karunaratne E, Niemann S, Perera J, Köser CU, et al. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype composed of overlapping MIC distributions with different underlying resistance mechanisms. Antimicrob Agents Chemother. 2019;63:e00092–19. DOIPubMedGoogle Scholar
- Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:139–45.PubMedGoogle Scholar
- Richardson M, Kirkham J, Dwan K, Sloan DJ, Davies G, Jorgensen AL. NAT2 variants and toxicity related to anti-tuberculosis agents: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23:293–305. DOIPubMedGoogle Scholar
Figure
Table
Suggested citation for this article: Gerussi V, Petersen T, Bonnet I, Aubry A, Bachir M, Gyde E, et al. Evaluation of high-dose isoniazid in multidrug-resistant tuberculosis treatment. Emerg Infect Dis. 2025 Mar [date cited]. https://doi.org/10.3201/eid3103.241473
Original Publication Date: February 21, 2025
Table of Contents – Volume 31, Number 3—March 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Valentina Gerussi, Infectious and Tropical Diseases Unit, Trieste University Hospital, Via Giuseppe Lorenzo Gatteri 25/1, 34125 Trieste, Italy
Top