Volume 31, Number 6—June 2025
Research Letter
Avian Influenza A(H5N1) Isolated from Dairy Farm Worker, Michigan, USA
Cite This Article
Citation for Media
Abstract
Influenza A(H5N1) viruses have been detected in US dairy cow herds since 2024. We assessed the pathogenesis, transmission, and airborne release of A/Michigan/90/2024, an H5N1 isolate from a dairy farm worker in Michigan, in the ferret model. Results show this virus caused airborne transmission with moderate pathogenicity, including limited extrapulmonary spread, without lethality.
Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b viruses have displayed unprecedented global spread among wild birds leading to numerous spillover infections in mammalian species. Of note, outbreaks in dairy cattle and gallinaceous birds have resulted in human infections in the United States during 2024–2025 (1). Increased frequency of H5N1 viruses crossing species barriers has caused concern that the avian influenza viruses are adapting to mammals. A critical component of influenza pandemic preparedness is early identification of emerging novel influenza viruses that cause disease and transmit efficiently in humans. A clade 2.3.4.4b H5N1 virus, A/Michigan/90/2024 (MI90), genotype B3.13, was isolated from a conjunctival swab specimen collected from a human patient in Michigan with conjunctivitis after exposure to infected cattle (2,3). In this article, we report the pathogenesis, transmission, and airborne exhalation of MI90 virus in ferrets, the standard animal model for influenza virus risk assessments (4).
We inoculated 18 ferrets with MI90 virus as previously described (5,6). We euthanized 3 ferrets on 3 and 5 days postinoculation (dpi) to assess virus spread in tissues. We used 6 ferrets to assess transmission in a cohoused, direct contact setting as a direct contact transmission model and through the air in the absence of direct or indirect contact as a respiratory droplet transmission model. We paired each ferret with a naive contact, as previously described (4). We observed clinical manifestations daily and collected nasal wash (NW), conjunctival, and rectal swab samples every 2 days postinoculation or postcontact. We confirmed transmission by testing for seroconversion to homologous virus in the contact animals.
Although all MI90-infected ferrets survived the 21-day study, we noted moderate disease. In inoculated ferrets, the mean maximum weight loss was 9.8%, fever (1.8°C above baseline) and lethargy were transient, and nasal and ocular discharge and sneezing were evident at 4–9 dpi (Table). We detected virus 3 dpi primarily in respiratory tract tissues; titers were highest in ethmoid turbinate samples (7.4 log10 PFU/mL) and at low levels in brain and gastrointestinal tissues. We observed similar results in tissues collected 5 dpi.
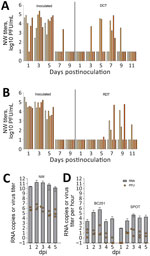
During the direct contact transmission experiment, inoculated ferrets shed virus in NW that peaked at 4.7–5.4 log10 PFU/mL at 1–5 dpi (Figure, panel A). Four of 6 cohoused contact animals had virus in NW (peak 2.5–4.9 log10 PFU/mL) at 5–7 days postcontact, whereas all 6 contact animals had viral RNA detected (3.6–7.7 log10 copies/mL) in NW (7) and seroconverted to MI90 virus, indicating that transmission was 100% (6/6 animals). In the respiratory droplet transmission experiment, NW collected from inoculated animals peaked 2.6–4.8 log10 PFU/mL at 1–3 dpi, whereas 3/6 contact ferrets had detectable virus in NW by day 7 postcontact (peak 2.6–4.8 log10 PFU/mL; days 9–11 postcontact) (Figure, panel B) as well as viral RNA (6.7–8.2 log10 copies/mL), and seroconverted, confirming transmission through the air in 50% of ferrets (3/6). We also detected infectious virus in conjunctival and rectal samples from inoculated animals, but only from 2 contact animals (Table).
To further evaluate the level of virus exhaled by MI90-inoculated ferrets and the potential for airborne transmission, we collected aerosol samples 1 time each day at 1–5 dpi for 1 hour from the 3 ferrets that were euthanized at 5 dpi. Air samples were analyzed for infectious virus and viral RNA by using the BC251 cyclone-based sampler (kindly provided by Dr. William Lindsley, National Institute for Occupational Safety and Health) and the SPOT water condensation sampler (Aerosol Devices, https://aerosoldevices.com), as described previously (8) (Figure, panel D). The highest mean titer of virus was detected at 2 dpi in NW collected from all 3 inoculated ferrets (6.5 log10 PFU/mL) (Figure, panel C). Airborne virus was highest at 3 dpi as measured in both samplers, up to 133 and 41 PFU/hour, supporting transmission observed in both contact models within 3–5 days after exposure.
Overall, MI90 virus displayed reduced virulence in ferrets compared with another H5N1 virus isolated from a dairy farm worker in Texas (8,9); the Texas virus possesses a genetic marker in the polymerase basic 2 protein (E627K), known for enhanced replication and pathogenesis in mammals. At this position, MI90 encodes 627E, like most other viruses isolated from cattle, and contains polymerase basic 2 M631L, which is associated with mammal adaptation (3,9). In addition, polymerase acidic 142N/E has been linked to increased virulence in mice (10); the Texas virus has an E and MI90 virus has a K at this position. Both viruses have identical hemagglutinin sequences associated with receptor binding and the multi-basic cleavage site. Despite differences in virulence, both viruses transmitted in the ferret model with similar proficiency and levels of airborne virus.
Because avian H5N1 viruses cross the species barrier and adapt to dairy cattle, each associated human infection presents further opportunity for mammal adaption. This potential poses an ongoing threat to public health and requires continual surveillance and risk assessment of emerging viruses to improve our ability to predict and prepare for the next influenza pandemic.
Dr. Brock is a microbiologist in the Influenza Division, National Center for Immunization and Respiratory Diseases, at the Centers for Disease Control and Prevention. Her research interests include the pathogenicity, transmissibility, and host response associated with emerging strains of influenza virus.
Acknowledgments
We thank the Centers for Disease Control and Prevention’s Comparative Medicine Branch (Division of Scientific Resources, National Center for Emerging and Zoonotic Infectious Diseases) for providing excellent animal care and the Mid-Michigan District Health Department and the Michigan Department of Health and Human Services for access to the human samples for virus isolation. We thank William Lindsley for his extensive contributions in developing and generously providing access to BC251 cyclone samplers, which have led to risk assessments of airborne pathogens worldwide.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Centers for Disease Control and Prevention and were conducted in an AAALAC-accredited facility.
References
- Webby RJ, Uyeki TM. An update on highly pathogenic avian influenza A(H5N1) virus, clade 2.3.4.4b. J Infect Dis. 2024;230:533–42. DOIPubMedGoogle Scholar
- Garg S, Reed C, Davis CT, Uyeki TM, Behravesh CB, Kniss K, et al. Outbreak of highly pathogenic avian influenza a(h5n1) viruses in U.S. dairy cattle and detection of two human cases—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73:501–5. DOIPubMedGoogle Scholar
- Morse J, Coyle J, Mikesell L, Stoddard B, Eckel S, Weinberg M, et al. Influenza A(H5N1) virus infection in two dairy farm workers in Michigan. N Engl J Med. 2024;391:963–4. DOIPubMedGoogle Scholar
- Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A. 2006;103:12121–6. DOIPubMedGoogle Scholar
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–800. DOIPubMedGoogle Scholar
- Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 2012;8:
e1002569 . DOIPubMedGoogle Scholar - Pulit-Penaloza JA, Brock N, Belser JA, Sun X, Pappas C, Tumpey TM, et al. Kinetics and magnitude of viral RNA shedding as indicators for Influenza A virus transmissibility in ferrets. Commun Biol. 2023;6:90. DOIPubMedGoogle Scholar
- Pulit-Penaloza JA, Belser JA, Brock N, Kieran TJ, Sun X, Pappas C, et al. Transmission of a human isolate of clade 2.3.4.4b A(H5N1) virus in ferrets. Nature. 2024;636:705–10. DOIPubMedGoogle Scholar
- Gu C, Maemura T, Guan L, Eisfeld AJ, Biswas A, Kiso M, et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature. 2024;636:711–8. DOIPubMedGoogle Scholar
- Kim JH, Hatta M, Watanabe S, Neumann G, Watanabe T, Kawaoka Y. Role of host-specific amino acids in the pathogenicity of avian H5N1 influenza viruses in mice. J Gen Virol. 2010;91:1284–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: May 02, 2025
Table of Contents – Volume 31, Number 6—June 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Taronna R. Maines, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H17-5, Atlanta, GA 30329-4018, USA
Top