Volume 31, Number 6—June 2025
Research Letter
Avian Influenza A(H5N1) Isolated from Dairy Farm Worker, Michigan, USA
Figure
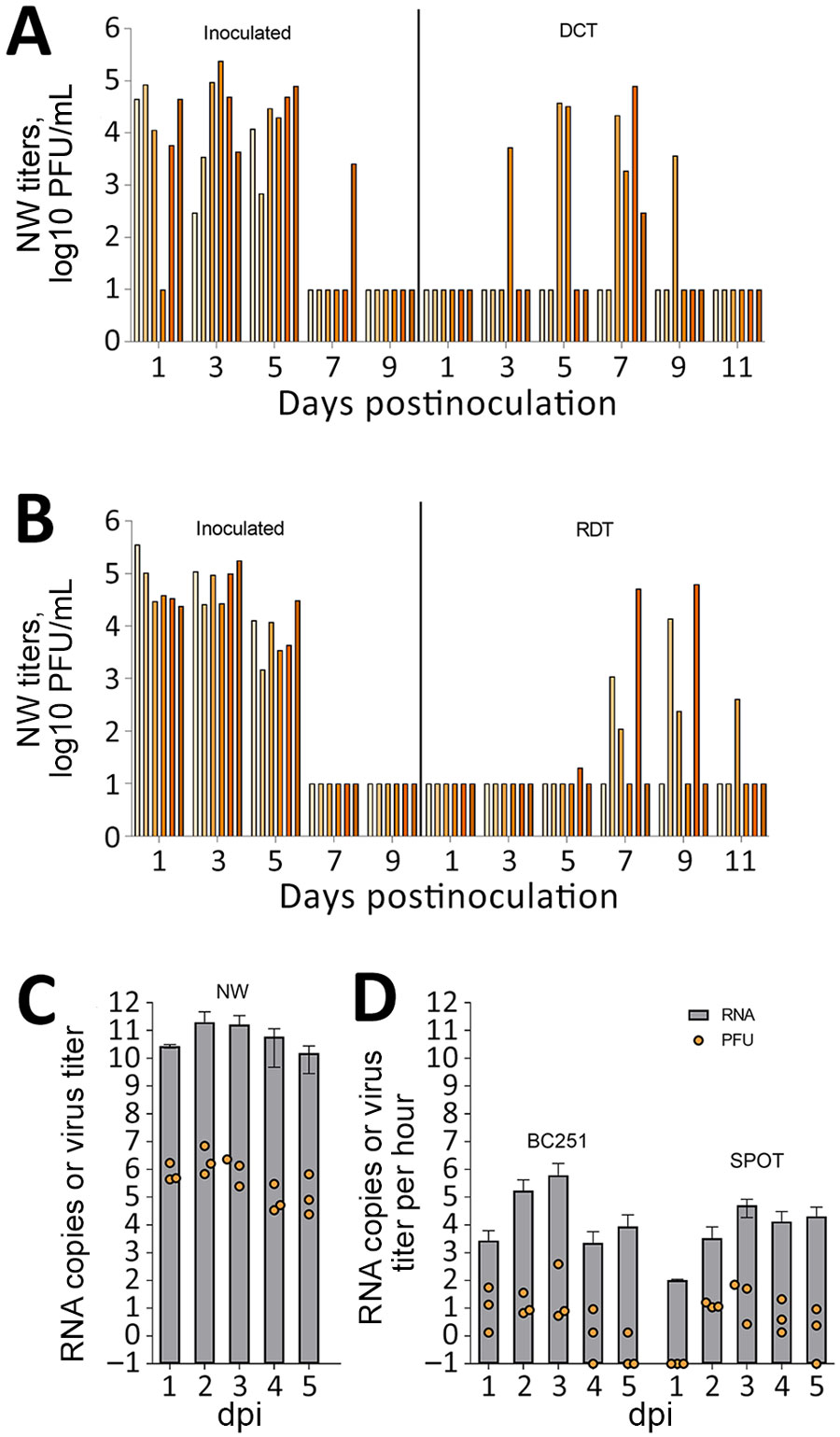
Figure. Transmission and measurement of airborne avian influenza A(H5N1) virus isolated from dairy farm worker, Michigan. A, B) For DCT and RDT testing, ferrets (n = 12) were intranasally inoculated with 106 PFU A/Michigan/90/2024 virus, isolated from the dairy worker, in 1 mL phosphate-buffered saline and were cohoused with naive ferrets in a DCT model (A) or in adjacent cages with perforated sidewalls permitting airborne virus spread but restricting contact in an RDT model (B). Each bar represents a single animal. C, D) For aerosol transmission testing, ferrets (n = 3) were inoculated intranasally with 106 PFU of MI90 virus and tested daily (C). Orange dots represent viral titers from NW in log10 PFU/mL; limit of detection 10 PFU/mL. Gray bars show average viral M gene RNA load. Error bars indicate SD. Limit of detection was 2.9 log10 RNA copies/mL. D) Aerosol samples were collected daily for 5 dpi by using a BC251 cyclone-based sampler (kindly provided by Dr. William Lindsley, National Institute for Occupational Safety and Health) and the SPOT water condensation sampler (Aerosol Devices, https://aerosoldevices.com), as described previously (8). Orange dots represent log10 PFU/mL per hour. Gray bars show average viral M gene RNA. Error bars indicate SD. Limit of detection was 2.5 log10 RNA copies/h. Ferrets were used for tissue collection on day 5. DCT, direct contact transmission; dpi, days postinoculation; NW, nasal washes; RDT, respiratory droplet transmission.
References
- Webby RJ, Uyeki TM. An update on highly pathogenic avian influenza A(H5N1) virus, clade 2.3.4.4b. J Infect Dis. 2024;230:533–42. DOIPubMedGoogle Scholar
- Garg S, Reed C, Davis CT, Uyeki TM, Behravesh CB, Kniss K, et al. Outbreak of highly pathogenic avian influenza a(h5n1) viruses in U.S. dairy cattle and detection of two human cases—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73:501–5. DOIPubMedGoogle Scholar
- Morse J, Coyle J, Mikesell L, Stoddard B, Eckel S, Weinberg M, et al. Influenza A(H5N1) virus infection in two dairy farm workers in Michigan. N Engl J Med. 2024;391:963–4. DOIPubMedGoogle Scholar
- Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A. 2006;103:12121–6. DOIPubMedGoogle Scholar
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–800. DOIPubMedGoogle Scholar
- Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 2012;8:
e1002569 . DOIPubMedGoogle Scholar - Pulit-Penaloza JA, Brock N, Belser JA, Sun X, Pappas C, Tumpey TM, et al. Kinetics and magnitude of viral RNA shedding as indicators for Influenza A virus transmissibility in ferrets. Commun Biol. 2023;6:90. DOIPubMedGoogle Scholar
- Pulit-Penaloza JA, Belser JA, Brock N, Kieran TJ, Sun X, Pappas C, et al. Transmission of a human isolate of clade 2.3.4.4b A(H5N1) virus in ferrets. Nature. 2024;636:705–10. DOIPubMedGoogle Scholar
- Gu C, Maemura T, Guan L, Eisfeld AJ, Biswas A, Kiso M, et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature. 2024;636:711–8. DOIPubMedGoogle Scholar
- Kim JH, Hatta M, Watanabe S, Neumann G, Watanabe T, Kawaoka Y. Role of host-specific amino acids in the pathogenicity of avian H5N1 influenza viruses in mice. J Gen Virol. 2010;91:1284–9. DOIPubMedGoogle Scholar