Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 7—July 2025
Research
Emergence of Flucytosine-Resistant Candida tropicalis Clade, the Netherlands
Suggested citation for this article
Abstract
Candida tropicalis is the second most virulent Candida species after C. albicans. Previous studies from the Netherlands and France reported a notable reduction in susceptibility to flucytosine (5-FC) in a substantial proportion of C. tropicalis isolates. We investigated epidemiologic patterns of C. tropicalis isolates in the Netherlands and the genetic mechanisms driving widespread non–wild-type (WT) 5-FC resistance. We conducted antifungal susceptibility testing and used advanced molecular techniques, including short tandem repeat genotyping and whole-genome sequencing paired with single-nucleotide polymorphism analysis, to analyze 250 C. tropicalis isolates collected across the Netherlands during 2012–2022. Our findings revealed the rapid emergence of a 5-FC–resistant, non-WT C. tropicalis clade, accounting for >40% of all C. tropicalis isolates by 2022. Genomic analysis identified a homozygous nonsense mutation in the FCY2 gene, which was exclusive to this non-WT population. Continued surveillance efforts are needed to detect and prevent the spread of drug-resistant Candida species.
Candida tropicalis is among the 5 most common Candida species found in healthcare settings (1–3). This diploid yeast is prevalent in Latin America and Asia and is occasionally reported in Africa and Europe (3,4). Since the 2000s, C. tropicalis has emerged as a substantial cause of candidemia, particularly in patients with neutropenia (5). C. tropicalis is considered the second most virulent Candida species, after C. albicans (6). Antifungal drug resistance has been increasingly reported for C. tropicalis, especially resistance to azoles (7,8) and, to a lesser degree, amphotericin B and echinocandins (9,10). The World Health Organization lists C. tropicalis as a high-risk pathogen (11,12), underscoring its considerable threat to public health and the need for research and surveillance.
Echinocandins are the first-line treatment for candidemia. Flucytosine, also known as 5-fluorocytosine (5-FC), is used to treat severe invasive candidiasis, which can cause endocarditis, endophthalmitis, or meningitis (13,14). Although 5-FC is only used in combination therapy because of the rapid emergence of isolates with high MICs (14), it shows potent activity against most yeasts, including C. tropicalis (15). Global rates of 5-FC–resistant, non–wild-type (WT) C. tropicalis are low, at ≈10% (15,16). However, we previously found a high percentage of C. tropicalis isolates with increased 5-FC MICs in the Netherlands (17), which has also been observed in France, where susceptibility to 5-FC has been documented in non-WT C. tropicalis since the 1980s (18). A 4-year survey conducted during 2002–2006 in the Paris area revealed increased 5-FC MICs in 45 (35%) of 130 C. tropicalis isolates recovered from blood cultures. Specific genetic mutations in the URA3 gene were observed in all isolates with increased 5-FC MICs. In addition, the non-WT strains shared identical multilocus sequence typing (MLST) genotypes, indicating clonal spread (18).
To investigate the recent decrease in 5-FC susceptibility in C. tropicalis isolates in the Netherlands, we performed a literature review and used available epidemiologic data from the Radboud University Medical Center CWZ Center of Expertise for Mycology (Nijmegen, the Netherlands). We applied a newly developed, highly reproducible short tandem repeat (STR) assay and whole-genome sequencing (WGS) to genotype C. tropicalis isolates (19,20), describe the epidemiology of C. tropicalis isolates over time, and identify the genetic basis of the non-WT 5-FC–resistant phenotype. Because the data consisted solely of information about clinical strains and did not include patient details, no ethics approval was required according to local guidelines.
Literature Review
To obtain an updated overview of 5-FC resistance in C. tropicalis, we conducted a comprehensive literature search across electronic databases, including PubMed and Google Scholar, by using the keywords “C. tropicalis,” “5-FC/flucytosine/fluorocytosine resistance” and “clonal resistance in C. tropicalis.” We also reviewed citations within the retrieved studies. We identified 15 relevant studies and extracted data on geographic region, time period, number of isolates, antifungal susceptibility testing (AFST) methods, interpretation criteria, and rates of non-WT 5-FC resistance.
Clinical Isolate Collection
A total of 250 nonreplicated clinical isolates of C. tropicalis were collected from patients across the Netherlands during January 2012–May 2022. The sources of isolates were as follows: blood, other sterile sites, oropharynx (including sputum and bronchoalveolar lavage samples), vagina, feces, urine, and other superficial sources. We identified Candida spp. by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker, https://www.bruker.com). We stored isolates at −70°C in 10% glycerol and grew them on Sabouraud dextrose agar plates at 30°C for 2–5 days before testing.
Antifungal Susceptibility Testing
We determined MICs for 5-FC and 9 other antifungal drugs (fluconazole, voriconazole, itraconazole, posaconazole, miconazole, amphotericin B, anidulafungin, caspofungin, and micafungin) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) E.def 7.4 microdilution method (21). We established local epidemiologic cutoffs (ECOFFs) for 5-FC by using the eyeballing method (17) and classified isolates with a 5-FC MIC of >0.5mg/L local ECOFF as non-WT. For fluconazole, we classified isolates with MICs >1 mg/L ECOFF as non-WT. We defined resistance by using EUCAST breakpoints (version 5.0) as follows: fluconazole, >4 mg/L; itraconazole, >0.125 mg/L; voriconazole, >0.25 mg/L; posaconazole, >0.06 mg/L; anidulafungin, >0.06 mg/L; micafungin, >0.06 mg/L; and amphotericin B, >1 mg/L.
DNA Extraction and STR Genotyping
We extracted DNA from the isolates after 24 hours of incubation on Sabouraud agar. We suspended single colonies in 1 mL of distilled water in a microcentrifuge tube and extracted DNA by using the High Pure PCR Template Preparation Kit (Roche Diagnostics, https://www.roche.com) according to manufacturer instructions. We genotyped the isolates by PCR amplifying and analyzing 6 STR markers, as previously described (20).
WGS and Single-Nucleotide Polymorphism Analysis
We selected 16 C. tropicalis isolates for WGS, including five 5-FC–resistant, non-WT isolates that clustered in 1 clade according to STR genotyping, 3 non-WT isolates that grouped outside the clade, and 8 phenotypically WT isolates. We extracted DNA by using InstaGene Matrix (Bio-Rad Laboratories, https://www.bio-rad.com) and sequenced by using Illumina technology (Illumina, https://www.illumina.com). Initially, we added 200 µL of InstaGene Matrix to the yeast pellets, vortexed at 500 rpm, and incubated for 30 min at 56°C, followed by another 30 min at 99°C. We then transferred samples to tubes containing glass beads with a particle size of <106 μm (Sigma Aldrich, https://www.sigmaaldrich.com) and conducted 2 rounds of bead beating at 17,000 rpm by using a MagNA Lyser (Roche Diagnostics). We assessed DNA integrity by using a TapeStation 2200 system (Agilent, https://www.agilent.com) and measured DNA concentrations by using a Qubit fluorometer (Thermo Fisher Scientific, https://www/thermofisher.com). We prepared libraries by using the Nextera DNA Flex kit (Illumina) following the manufacturer’s instructions. We performed paired-end, 2 × 150-bp mode sequencing on an Illumina NextSeq 550 system (Illumina).
We compared isolate sequences to C. tropicalis sequences retrieved from the National Center for Biotechnology Information Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) (Appendix Table 1). We performed WGS single-nucleotide polymorphism (SNP) analysis by using Illumina reads, as previously described (19). We aligned reads with the C. tropicalis reference genome MYA-3404 (SRA accession no. GCA_013177555.1) by using BWA-MEM version 0.7.17 (https://github.com/j-levy/bwa) and subsequently filtered to remove duplicates, unpaired reads, and reads with MAPQ scores <60. We detected SNPs by using FreeBayes version 1.3.6 (https://github.com/freebayes/freebayes) and removed SNPs with a read depth of <25, quality of <100, allele frequency between 0.15 × depth and 0.45 × depth, and allele frequency between 0.55 × depth and 0.9 × depth. We performed phylogenetic analysis by using VCF2PopTree (https://github.com/sansubs/vcf2pop), MEGA11 version 11.0.10 (https://www.megasoftware.net), and iTOL version 6 (https://itol.embl.de) for visualization. We located the resistance-associated genes FUR1 (GenBank accession no. EU327981.1), FCY1 (accession no. EU327982.1), FCY2 (accession no. HQ166001.1), and URA3 (accession no. EU288195.1) within the reference genome MYA-3404 and inspected those for missense mutations by using IGV version 2.17.3 (22). We assessed copy number variation and large-scale deletions by using YMAP (23) for all C. tropicalis assemblies, as well as for the MYA-3404 reference strain.
URA3 Gene Sequencing
We investigated whether the mechanism underlying 5-FC resistance was related to the URA3 gene mutation resulting in a K177E amino acid substitution (18). We sequenced the URA3 gene from six 5-FC WT (susceptible) and 24 randomly selected 5-FC–resistant non-WT C. tropicalis isolates. We grew the isolates on yeast extract/peptone/dextrose agar plates for 24 hours at 30°C and used a standard DNA extraction protocol (24). We transferred cells to 1.5 mL tubes containing 600 µL glass beads (diameter 0.4–0.6 mm) and 250 µL breaking buffer (2% Triton X-100, 1% sodium dodecyl sulfate, 2 mol/L NaCl, 1 mol/L Tris-HCl pH 8, 0.5 mol/L EDTA pH 8, and Milli-Q water), shook the tubes for 30 minutes at 60°C, and centrifuged them at 1,000 × g. After centrifugation, we added 700 µL of phenol/chloroform/isoamyl alcohol and shook the mixture for 5 minutes at room temperature. We centrifuged the tubes at 10,000 × g for 5 minutes and then transferred the resulting upper layer to a new tube and stored at 20°C (room temperature) until analysis. We amplified the URA3 gene by using the PCR primers and methods described previously (25). All sequence data generated in this study were deposited in the SRA database (BioProject accession nos. PRJNA1090665, PRJNA110750, PRJNA1107503).
Epidemiologic Analysis and Data Analysis
We analyzed epidemiologic data from the Radboud University Medical Center laboratory information system to assess the distribution of 5-FC non-WT isolates over time and evaluated temporal trends. We plotted the annual rates of 5-FC non-WT isolates and performed linear regression analysis by using GraphPad Prism (GraphPad, https://www.graphpad.com). We considered the trend to be significant if the slope deviated from zero (p<0.05). We also examined correlations between 5-FC and fluconazole resistance.
Literature Review
We retrieved 15 studies describing C. tropicalis resistance to 5-FC and summarized those data (Table). Most (n = 12) studies were published during 2000–2012. Data on C. tropicalis have been reported from various regions, including global collections and countries, such as the United States, United Kingdom, Japan, South Korea, France, Italy, Spain, and Germany. The Clinical and Laboratory Standards Institute broth microdilution method (38) was used predominantly (8 studies) to test C. tropicalis isolates, followed by the EUCAST reference broth microdilution method, and then E-test gradient strips, ATB Fungus, and VITEK automated susceptibility testing (all bioMérieux, https://www.biomerieux.com).
The frequency of C. tropicalis isolates with elevated MICs to 5-FC ranged from 0%–10% (Italy, Spain, South Korea) to 10%–30% (including studies from Spain, United States, and United Kingdom) in global collections and was >30% in France and Germany. A genetic survey of non-WT C. tropicalis isolates from blood samples in Paris, collected during 2002–2006, identified a group of non-WT isolates with the same MLST profile, all having a URA3K177E mutation (18). Epidemiologic analysis indicated that the group of non-WT clones frequently caused candidemia in patients with malignancies and was associated with better outcomes; recurrent spread was noted during the study period. Genetic relatedness of 5-FC non-WT isolates from specific clades in the United Kingdom (2002–2003) and Belgium (1998) has also been suggested (39). The spread of the clade from Paris to other regions of France and other countries in Europe has not been investigated further.
Antifungal Drug Susceptibility Testing
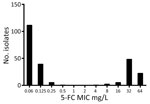
To gain more insight into the susceptibility of C. tropicalis isolates to 5-FC in the Netherlands, we performed AFST on 250 clinical strains, for which the isolation source of 104 isolates was available, by using the EUCAST microbroth dilution (Appendix Table 2). The modal 5-FC MIC was 0.06 mg/L, and local ECOFF was 0.5 mg/L (Figure 1). C. tropicalis isolates displayed a bimodal distribution; we observed a clear separation of 2 subpopulations. The first subpopulation consisted of 168 (67.2%) isolates with low 5-FC MICs (<0.5 mg/L) and were classified as WT, whereas the second subpopulation consisted of 82 (32.8%) isolates with high 5-FC MICs (>0.5 mg/L) and were classified as non-WT. AFST of 9 additional antifungal agents did not show that typical bimodal distribution (Appendix Table 3). All isolates were susceptible to amphotericin B. Resistance rates (EUCAST clinical breakpoints) were as follows: fluconazole, 11.3% (28/248); itraconazole, 7.3% (7/95); voriconazole, 8.8% (22/249); posaconazole, 7.7% (4/52); and anidulafungin, 0.8% (2/246). We did not interpret MIC data for miconazole, caspofungin, and micafungin because of the lack of EUCAST clinical breakpoints for those drugs. Overall, 25 (10%) isolates exhibited cross-resistance. We did not observe a correlation between 5-FC non-WT and azole resistance.
Phylogenetic Analysis using STR
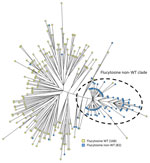
We performed STR genotyping for all 250 isolates. Except for 2 isolates (nos. v139–74 and v267–58), which had identical genotypes, all isolates displayed a unique genotype (Appendix Figure). Of the 82 isolates with the 5-FC non-WT phenotype, 65 (79.3%) were closely related and formed a distinct clade, whereas the remaining non-WT isolates did not cluster (Figure 2). Isolates within this clade differed by <4 STR markers; high variability occurred in the first and second markers of the PCR M6 panel, and little variation occurred in PCR M3a and M3b panels (Appendix Figure) (20). In contrast, isolates outside this clade differed in >4 markers, usually 5 or 6, and had larger copy number differences. Non-WT strains within the distinct clade containing genotypes 84–147 were isolated during the entire study period.
WGS Analysis
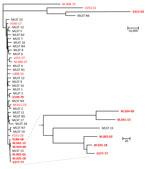
To validate the inferred genetic relatedness, STR outcomes were compared with WGS SNP calling. Isolates that were part of the non-WT clade, according to the STR data (n = 5), also clustered according to WGS SNP analysis (Figure 3). Within the non-WT clade, the genetic diversity was relatively low (304/2,289 SNPs), whereas isolates outside the clade displayed >20,000 SNPs compared with the most related isolate. Isolate v186-48 was most closely related to the non-WT clade (according to 19,365 SNPs). The remaining 5-FC non-WT isolates, which were not located in this clade, did not cluster. To assess the global dispersal of the resistant clade, we compared the 5 isolates from the Netherlands belonging to the 5-FC non-WT clade, together with the other isolates from the Netherlands (from the WGS data), with 27 previously reported C. tropicalis MLST clades. Using WGS SNP analysis, including 1 representative isolate from each MLST clade, we found five 5-FC non-WT isolates from the Netherlands were most closely related to C. tropicalis MSLT 15 (Figure 4). The other isolates from the Netherlands formed a distinct branch.
Molecular Mechanisms of 5-FC Resistance
To investigate the mechanism of 5-FC resistance, we sequenced the URA3 gene of 30 isolates (6 WT and 24 non-WT) and inspected it for substitutions. The URA3K177E mutation occurred in all 5-FC non-WT isolates. However, two 5-FC susceptible isolates (M.040-37 and v252-37) also exhibited this mutation (either heterozygous or homozygous mutation), which we confirmed by visual inspection of WGS reads (Appendix Table 4). Therefore, we analyzed the resistance-associated genes FUR1, FCY1, FCY2, and URA3 by using available WGS data. In FCY2, the mutation causing an E49X amino acid nonsense substitution was homozygous in all isolates from the non-WT clade, whereas that mutation was either absent or heterozygous in isolates outside the clade (40). For the other resistance-associated genes, no missense mutations were exclusively present in the non-WT isolates. Subsequently, we assessed the isolates for copy number variation by using YMAP (23) and compared them with the MYA-3404 reference genome. For FUR1, FCY1, FCY2, and URA3, we found no copy number variation in the non-WT clade when compared with the 5-FC–susceptible isolates.
Epidemiology of 5-FC Non-WT clade
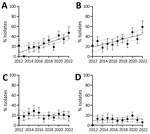
To elucidate the emergence of 5-FC non-WT C. tropicalis isolates over time, we compared those isolates with fluconazole non-WT and fluconazole-resistant isolates collected during 2012–2022 (Figure 5). The percentage and clustering of 5-FC–resistant non-WT isolates both increased during this timeframe, particularly since 2018; a substantial peak indicating a higher prevalence in the non-WT population occurred in 2022. In contrast, we observed no downward or upward trend for the fluconazole non-WT or resistant isolates.
We conducted a comprehensive analysis of 5-FC resistance in and genetic relatedness of clinical C. tropicalis isolates collected in the Netherlands during 2012–2022. The analyses showed a recent and substantial emergence of non-WT, 5-FC–resistant C. tropicalis isolates across the Netherlands since the 2010s. STR typing and WGS identified a circulating non-WT 5-FC–resistant clade; a marked increase in the percentage of isolates belonging to this clade was observed, particularly since 2018.
Approximately one third (32.4%) of the C. tropicalis isolates in our collection comprised non-WT strains resistant to 5-FC with a bimodal MIC distribution, indicating a heterogeneous population. Most 5-FC–resistant non-WT isolates had a MIC of 32 mg/L (Figure 1). STR genotyping demonstrated that ≈80% of non-WT isolates were genetically related and formed a distinct clade. The genetic diversity within that clade was low (304–2,289 SNP differences) but distinct, indicating past diversification and environmental spread rather than recent clonal transmission. The increasing prevalence of this clade suggests better adaptation compared with other C. tropicalis strains in the Netherlands, although changes in clinical practices and referral patterns might confound this observation.
Our study raises questions regarding the relationship between the 5-FC non-WT clade identified in the Netherlands and similar clades reported in other countries of Europe. A comparison of our WGS data with those of publicly available C. tropicalis isolates indicated the clade from the Netherlands is closely related to C. tropicalis MLST clade 15 from Denmark (41,42). A previous global study of 1,571 C. tropicalis isolates identified 10 isolates from MLST clade 15 (42), which included isolates from Denmark (n = 1) (41), Ireland (n = 1) (43), United Kingdom (n = 2), Belgium (n = 2), Taiwan (n = 1), and Thailand (n = 3) (42). The isolates from Belgium, cultured in 1993 and 1994, had 5-FC MICs of 128 mg/L. The UK isolates, cultured in 2004, had 5-FC MICs of 32 and 64 mg/L. The 5-FC MICs of the isolate from Taiwan cultured in 2006 and those from Denmark (date unknown) and Ireland (cultured in 2018) remain unknown (41,43). Several isolates from this study clustered with isolates from Belgium and the United Kingdom that had increased 5-FC MICs, indicating spread across Europe (39). The MLST 15 isolates from Thailand, cultured during 2015–2017, had 5-FC MICs of <0.5 mg/L (44), suggesting intraclade variation in 5-FC susceptibility.
In this study, we analyzed C. tropicalis isolates collected during 2012–2022. We conducted an earlier study, which included isolates cultured before 2012 (17). In that study, all 18 isolates tested during 2008–2010 were phenotypically 5-FC WT. In 2011, a total of 24 isolates were tested, 6 of which were 5-FC–resistant non-WT isolates. However, genetic analysis of those isolates has not been performed. All isolates collected since 2012 have been genotyped, and the first non-WT isolate from the clade in the Netherlands was identified in 2012 (Figure 5). In parallel with our study, investigators in Denmark also showed a marked increase in 5-FC–resistant, non-WT C. tropicalis isolates since the 2010s (40). In contrast, 5-FC–resistant non-WT isolates were documented in France as early as the 1980s (18), suggesting a later introduction or evolution of the 5-FC–resistant, non-WT clade in the Netherlands. Previous studies using MLST in the United Kingdom, Belgium, and France have also indicated the presence of non-WT C. tropicalis clades (18,39), although the lack of full-genome sequences prevents direct comparisons with our findings.
No 5-FC–resistant, non-WT clade has been reported outside Europe. However, C. tropicalis has demonstrated the ability to spread globally, evidenced by the worldwide distribution of other C. tropicalis clades. Azole-resistant C. tropicalis clades have been documented worldwide (42). The fluconazole-resistant MLST clade 4, comprising 248 of 1,571 isolates, mainly originated from Asia, whereas fluconazole-resistant MLST clades 2 and N2 have a global distribution (42). Despite the confinement of 5-FC–resistant, non-WT C tropicalis to Europe, the widespread prevalence of MLST clades 2 and N2 suggests a potential for global dissemination, emphasizing the importance of vigilance and global surveillance.
We found that 32.4% of C. tropicalis isolates were 5-FC–resistant non-WT strains, which was higher than the 19% fluconazole-resistant non-WT and 11% fluconazole-resistant strains. Higher fluconazole resistance rates have been reported in lower and middle income countries, such as China (23.1%) (42), Algeria (31.6%) (45) and Egypt (37.5%) (19), where fluconazole is the primary treatment for invasive fungal infections. Most fluconazole-resistant isolates in our study were from the 5-FC WT population; only 2 isolates exhibited non-WT 5-FC MICs. Despite the rising rate of 5-FC–resistant non-WT isolates, a major national trend was not observed for rates of fluconazole-resistant non-WT isolates.
The factors driving the recent emergence of the 5-FC–resistant, non-WT clade in the Netherlands are unclear. Possible reasons are selective pressure from antifungal drugs or cancer treatments, such as 5-fluorouracil, and better adaptation to human hosts, leading to greater colonization and spread. Unlike azole resistance, which might be linked to extensive clinical or agricultural azole use, 5-FC is rarely used outside medical contexts and seldom prescribed for invasive candidiasis, making resistance development through drug exposure unlikely. Increased 5-FC MICs were not linked to resistance to other antifungal agents, such as fluconazole or echinocandins, and genotyping did not suggest a clonal outbreak. Therefore, the factors behind the rise of this 5-FC–resistant, non-WT C. tropicalis clade remain unknown, necessitating further investigation to elucidate mechanisms and prevent spread.
Although 5-FC resistance has been previously associated with the K177E amino acid substitution from a mutation in URA3, this association has not been confirmed through transformation studies (18). In our study, the K177E mutation was present in all sequenced 5-FC–resistant, non-WT clade isolates; however, it was also detected in non-WT isolates, indicating that this mutation alone cannot fully explain the non-WT resistant phenotype. In Denmark, a mutation in the FCY2 gene resulting in the E49X amino acid substitution was found in all 5-FC non-WT isolates, possibly overlooked previously because of an error in the FCY2 reference sequence (40). When checking for this mutation, we found it to be homozygous in the 5-FC clade isolates from the Netherlands, which has previously been shown to cause a 5-FC non-WT phenotype (34). That laboratory study exposed isolates heterozygous for this mutation to 5-FC and showed that those isolates developed a non-WT 5-FC–resistant phenotype because of loss of heterozygosity for that mutation (34). In addition, constructed strains homozygous for the truncated protein and for glutamic acid at amino acid position 49 indicated that the homozygous truncated protein caused a non-WT phenotype, whereas isolates with glutamic acid at position 49 were WT (34).
In conclusion, our study confirmed the presence of a 5-FC–resistant, non-WT clade in the Netherlands; similar trends were observed in Denmark (40), likely because of the same 5-FC non-WT clade. Further prospective studies are required to gain more epidemiologic insights and clarify the effect of this 5-FC non-WT clade on patient outcomes. Our findings highlight the importance of continuous surveillance, advanced genotyping techniques, and comprehensive clinical data collection to prevent the spread of drug-resistant Candida spp.
Ms. Delma is a PhD candidate at the Radboudumc-CWZ Center of Expertise for Mycology in Nijmegen, the Netherlands. Her primary research interests focus on flucytosine resistance in medically important fungi.
References
- McCarty TP, Pappas PG. Invasive Candidiasis. Infect Dis Clin North Am. 2016;30:103–24. DOIPubMedGoogle Scholar
- Lass-Flörl C, Kanj SS, Govender NP, Thompson GR III, Ostrosky-Zeichner L, Govrins MA. Invasive candidiasis. Nat Rev Dis Primers. 2024;10:20. DOIPubMedGoogle Scholar
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20(Suppl 6):5–10. DOIPubMedGoogle Scholar
- Dougue AN, El-Kholy MA, Giuffrè L, Galeano G, D Aleo F, Kountchou CL, et al. Multilocus sequence typing (MLST) analysis reveals many novel genotypes and a high level of genetic diversity in Candida tropicalis isolates from Italy and Africa. Mycoses. 2022;65:989–1000. DOIPubMedGoogle Scholar
- Chai LY, Denning DW, Warn P. Candida tropicalis in human disease. Crit Rev Microbiol. 2010;36:282–98. DOIPubMedGoogle Scholar
- Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An update on Candida tropicalis based on basic and clinical approaches. Front Microbiol. 2017;8:1927. DOIPubMedGoogle Scholar
- Spruijtenburg B, Baqueiro CCSZ, Colombo AL, Meijer EFJ, de Almeida JN Jr, Berrio I, et al.; On Behalf Of The Latin American Group For Investigating Candida Tropicalis Resistance. on behalf of the Latin American Group For Investigating Candida tropicalis Resistance. Short tandem repeat genotyping and antifungal susceptibility testing of Latin American Candida tropicalis isolates. J Fungi (Basel). 2023;9:207. DOIPubMedGoogle Scholar
- Paul S, Shaw D, Joshi H, Singh S, Chakrabarti A, Rudramurthy SM, et al. Mechanisms of azole antifungal resistance in clinical isolates of Candida tropicalis. PLoS One. 2022;17:
e0269721 . DOIPubMedGoogle Scholar - Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25:792–8. DOIPubMedGoogle Scholar
- Khan Z, Ahmad S, Mokaddas E, Meis JF, Joseph L, Abdullah A, et al. Development of echinocandin resistance in Candida tropicalis following short-term exposure to caspofungin for empiric therapy. Antimicrob Agents Chemother. 2018;62:e01926–17. DOIPubMedGoogle Scholar
- World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. October 25, 2022 [cited 2025 May 2]. https://www.who.int/publications/i/item/9789240060241
- Keighley C, Kim HY, Kidd S, Chen SC, Alastruey A, Dao A, et al. Candida tropicalis-A systematic review to inform the World Health Organization of a fungal priority pathogens list. Med Mycol. 2024;62:
myae040 . DOIPubMedGoogle Scholar - Sigera LSM, Denning DW. Flucytosine and its clinical usage. Ther Adv Infect Dis. 2023;10:
20499361231161387 . DOIPubMedGoogle Scholar - Cornely OA, Sprute R, Bassetti M, Chen SC, Groll AH, Kurzai O, et al. Global guideline for the diagnosis and management of candidiasis: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis. 2025;25:e280–93. DOIPubMedGoogle Scholar
- Delma FZ, Al-Hatmi AMS, Brüggemann RJM, Melchers WJG, de Hoog S, Verweij PE, et al. Molecular nechanisms of 5-fluorocytosine resistance in yeasts and filamentous fungi. J Fungi (Basel). 2021;7:909. DOIPubMedGoogle Scholar
- Pfaller MA, Messer SA, Boyken L, Huynh H, Hollis RJ, Diekema DJ. In vitro activities of 5-fluorocytosine against 8,803 clinical isolates of Candida spp.: global assessment of primary resistance using National Committee for Clinical Laboratory Standards susceptibility testing methods. Antimicrob Agents Chemother. 2002;46:3518–21. DOIPubMedGoogle Scholar
- Delma FZ, Melchers WJG, Verweij PE, Buil JB. Wild-type MIC distributions and epidemiological cutoff values for 5-flucytosine and Candida species as determined by EUCAST broth microdilution. JAC Antimicrob Resist. 2024;6:
dlae153 . DOIPubMedGoogle Scholar - Desnos-Ollivier M, Bretagne S, Bernède C, Robert V, Raoux D, Chachaty E, et al.; Yeasts Group. Clonal population of flucytosine-resistant Candida tropicalis from blood cultures, Paris, France. Emerg Infect Dis. 2008;14:557–65. DOIPubMedGoogle Scholar
- Spruijtenburg B, Meijer EFJ, Xiao M, Shawky SM, Meis JF, de Groot T, et al. Genotyping and susceptibility testing uncovers large azole-resistant Candida tropicalis clade in Alexandria, Egypt. J Glob Antimicrob Resist. 2023;34:99–105. DOIPubMedGoogle Scholar
- Spruijtenburg B, van Haren MHI, Chowdhary A, Meis JF, de Groot T. Development and application of a short tandem repeat multiplex typing assay for Candida tropicalis. Microbiol Spectr. 2023;11:
e0461822 . DOIPubMedGoogle Scholar - European Committee for Antimicrobial Susceptibility Testing. EUCAST Definitive Document E.Def 7.4. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. October 2023 [cited 2024 Mar 20]. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E.Def_7.4_Yeast_definitive_revised_2023.pdf
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6. DOIPubMedGoogle Scholar
- Abbey DA, Funt J, Lurie-Weinberger MN, Thompson DA, Regev A, Myers CL, et al. YMAP: a pipeline for visualization of copy number variation and loss of heterozygosity in eukaryotic pathogens. Genome Med. 2014;6:100. DOIPubMedGoogle Scholar
- Arentshorst M, Ram AF, Meyer V. Using non-homologous end-joining-deficient strains for functional gene analyses in filamentous fungi. Methods Mol Biol. 2012;835:133–50. DOIPubMedGoogle Scholar
- Dodgson AR, Dodgson KJ, Pujol C, Pfaller MA, Soll DR. Clade-specific flucytosine resistance is due to a single nucleotide change in the FUR1 gene of Candida albicans. Antimicrob Agents Chemother. 2004;48:2223–7. DOIPubMedGoogle Scholar
- Nerson D, De Closets F, Dupouy-Camet J, Kures L, Marjollet M, Poirot J, et al. Antifungal susceptibility of yeasts (and a few filamentous fungi) by a standardized micromethod [in French]. Bulletin de la Société Francaise de Mycologie Médicale. 1987;16:395–8.
- Law D, Moore CB, Joseph LA, Keaney MG, Denning DW. High incidence of antifungal drug resistance in Candida tropicalis. Int J Antimicrob Agents. 1996;7:241–5. DOIPubMedGoogle Scholar
- Barchiesi F, Arzeni D, Caselli F, Scalise G. Primary resistance to flucytosine among clinical isolates of Candida spp. J Antimicrob Chemother. 2000;45:408–9. DOIPubMedGoogle Scholar
- Cuenca-Estrella M, Díaz-Guerra TM, Mellado E, Rodríguez-Tudela JL. Flucytosine primary resistance in Candida species and Cryptococcus neoformans. Eur J Clin Microbiol Infect Dis. 2001;20:276–9. DOIPubMedGoogle Scholar
- Alexander BD, Byrne TC, Smith KL, Hanson KE, Anstrom KJ, Perfect JR, et al. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol. 2007;45:698–706. DOIPubMedGoogle Scholar
- Quindós G, Ruesga MT, Martín-Mazuelos E, Salesa R, Alonso-Vargas R, Carrillo-Muñoz AJ, et al. In-vitro activity of 5-fluorocytosine against 1,021 Spanish clinical isolates of Candida and other medically important yeasts. Rev Iberoam Micol. 2004;21:63–9.PubMedGoogle Scholar
- Takakura S, Fujihara N, Saito T, Kudo T, Iinuma Y, Ichiyama S. National surveillance of species distribution in blood isolates of Candida species in Japan and their susceptibility to six antifungal agents including voriconazole and micafungin. J Antimicrob Chemother. 2004;53:283–9. DOIPubMedGoogle Scholar
- Fleck R, Dietz A, Hof H. In vitro susceptibility of Candida species to five antifungal agents in a German university hospital assessed by the reference broth microdilution method and Etest. J Antimicrob Chemother. 2007;59:767–71. DOIPubMedGoogle Scholar
- Chen YN, Lo HJ, Wu CC, Ko HC, Chang TP, Yang YL. Loss of heterozygosity of FCY2 leading to the development of flucytosine resistance in Candida tropicalis. Antimicrob Agents Chemother. 2011;55:2506–14. DOIPubMedGoogle Scholar
- Lockhart SR, Bolden CB, Iqbal N, Kuykendall RJ. Validation of 24-hour flucytosine MIC determination by comparison with 48-hour determination by the Clinical and Laboratory Standards Institute M27-A3 broth microdilution reference method. J Clin Microbiol. 2011;49:4322–5. DOIPubMedGoogle Scholar
- Jung SI, Shin JH, Choi HJ, Ju MY, Kim SH, Lee WG, et al.; Korean Study Group for Candidemia. Antifungal susceptibility to amphotericin B, fluconazole, voriconazole, and flucytosine in Candida bloodstream isolates from 15 tertiary hospitals in Korea. Ann Lab Med. 2012;32:426–8. DOIPubMedGoogle Scholar
- Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. Potency of anidulafungin compared to nine other antifungal agents tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: results from the global SENTRY Antimicrobial Surveillance Program (2008). J Clin Microbiol. 2010;48:2984–7. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th edition (M27). Wayne (PA): The Institute; 2017.
- Tavanti A, Davidson AD, Johnson EM, Maiden MC, Shaw DJ, Gow NA, et al. Multilocus sequence typing for differentiation of strains of Candida tropicalis. J Clin Microbiol. 2005;43:5593–600. DOIPubMedGoogle Scholar
- Abou-Chakra N, Astvad KMT, Martinussen J, Munksgaard ASE, Arendrup MC. Exponential clonal expansion of 5-fluorocytosine–resistant C. tropicalis and new insights into underlying molecular mechanisms. Emerg Infect Dis. 2025;31:977–85. DOIPubMedGoogle Scholar
- Szarvas J, Rebelo AR, Bortolaia V, Leekitcharoenphon P, Schrøder Hansen D, Nielsen HL, et al. Danish whole-genome-sequenced Candida albicans and Candida glabrata samples fit into globally prevalent clades. J Fungi (Basel). 2021;7:962. DOIPubMedGoogle Scholar
- Fan X, Dai RC, Zhang S, Geng YY, Kang M, Guo DW, et al. Tandem gene duplications contributed to high-level azole resistance in a rapidly expanding Candida tropicalis population. Nat Commun. 2023;14:8369. DOIPubMedGoogle Scholar
- O’Brien CE, Oliveira-Pacheco J, Ó Cinnéide E, Haase MAB, Hittinger CT, Rogers TR, et al. Population genomics of the pathogenic yeast Candida tropicalis identifies hybrid isolates in environmental samples. PLoS Pathog. 2021;17:
e1009138 . DOIPubMedGoogle Scholar - Tulyaprawat O, Pharkjaksu S, Chongtrakool P, Ngamskulrungroj P. An association of an eBURST group with triazole resistance of Candida tropicalis blood isolates. Front Microbiol. 2020;11:934. DOIPubMedGoogle Scholar
- Megri Y, Arastehfar A, Boekhout T, Daneshnia F, Hörtnagl C, Sartori B, et al. Candida tropicalis is the most prevalent yeast species causing candidemia in Algeria: the urgent need for antifungal stewardship and infection control measures. Antimicrob Resist Infect Control. 2020;9:50. DOIPubMedGoogle Scholar
Figures
Table
Suggested citation for this article: Delma FZ, Spruijtenburg B, Meis JF, de Jong AW, Groot J, Rhodes J, et al. Emergence of flucytosine-resistant Candida tropicalis clade, the Netherlands. Emerg Infect Dis. 2025 Jul [date cited]. https://doi.org/10.3201/eid3107.241918
Original Publication Date: June 11, 2025
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Table of Contents – Volume 31, Number 7—July 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jochem B. Buil, Department of Medical Microbiology, Radboud University Medical Center, Geert grooteplein zuid 10, 6525 GA, Nijmegen, the Netherlands
Top