Volume 7, Number 2—April 2001
THEME ISSUE
4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections
State of the Art
New Technology for Detecting Multidrug-Resistant Pathogens in the Clinical Microbiology Laboratory
Cite This Article
Citation for Media
Abstract
Northwestern Memorial Hospital instituted in-house molecular typing to rapidly assess microbial clonality and integrated this typing into an infection control program. We compared data on nosocomial infections collected during 24 months before and 60 months after implementing the new program. During the intervention period, infections per 1,000 patient-days fell 13% (p=0.002) and the percentage of hospitalized patients with nosocomial infections decreased 23% (p=0.000006). In our hospital, the percentage of patients with nosocomial infections is 43% below the U.S. rate. Our typing laboratory costs approximately $400,000 per year, a savings of $5.00 for each dollar spent
The nosocomial infection rate in U.S. hospitals in the early 1980s was 5.7% (1). Two million Americans acquire a nosocomial infection each year (2), at a rate of 5 per 100 admissions (5%). These infections cost $4.5 billion annually, and 88,000 patients die from them each year; 70% of infections are due to organisms resistant to at least one antimicrobial agent. Although 1.8 million fewer patients were admitted to U.S. hospitals in 1995 than in 1975 (35.9 million versus 37.7 million) and the average length of stay was lower (5.3 days in 1995 vs. 7.9 days in 1975), the national nosocomial rate was increasing. In 1975, there were 7.18 nosocomial infections per 1,000 patient days compared to 9.77 in 1995, an increase of 36% (2).
Major nosocomial pathogens increasingly resistant to antimicrobial drugs include Escherichia coli, Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus species, and Pseudomonas aeruginosa (3,4). Infections from methicillin- resistant staphylococci, vancomycin-resistant enterococci (VRE), and aminoglycoside-resistant Pseudomonas spp. are becoming common (5,6).
The clinical laboratory has several critical roles in controlling hospital-acquired infections: accurately identifying nosocomial pathogens, detecting unexpected antimicrobial-drug resistance, and epidemiologic typing (7). Most new rapid tests are not yet helpful for infection control purposes, and automated systems for bacterial identification and susceptibility testing are not as reliable as desired for detecting organisms with emerging drug resistance (7). However, the laboratory can make key contributions through epidemiologic typing, particularly by collaborating with the infection control team during outbreak investigations (8). Molecular techniques for establishing the presence or absence of clonality can be very effective in tracking the spread of infections caused by genetically related pathogens (9–14).
We formed a permanent, integrated infection control and prevention program that fully incorporates infection control, infectious disease, pharmacy, and clinical microbiology personnel into a single working group to minimize hospital infections (15). We discuss our overall experience with such a program, which has been in place at Northwestern Memorial Hospital for more than 5 years. Our hospital, located in Chicago, is a 700-bed, university-affiliated medical center with more than 39,000 annual discharges, 56,000 emergency cases, and 260,000 annual outpatient visits. We initially postulated that our integrated infection control program could be medically and economically successful in minimizing the incidence of hospital-acquired infections. The laboratory's role was enhanced by introducing a molecular typing section within the Division of Clinical Microbiology; this section rapidly and systematically determines clonality and reports results immediately to the infection control practitioners so that they can quickly take appropriate action (3). We describe our experience with such a program after the first 60 months of its existence and compare its effect with the 24 months immediately before this expanded effort.
Nosocomial Infections
Nosocomial infections are detected by ongoing surveillance in intensive care units (ICUs), special-care nurseries, and post-surgery units. Standard infection definitions are used (16). The data we report represent the total number of nosocomial infections per 1,000 patient days, and the number of patients with nosocomial infections per 100 patient discharges (percentage of patients with nosocomial infection). Methods for data collection include review of microbiology reports and patients' medical records, direct observation of medical and nursing practice, active surveillance of rectal cultures of patients in nursing units for high-risk patients, and evaluation of suspected nosocomial infections reported by health-care providers. Three full-time infection control professionals collect the infection data. Interpretation, assessment, and planning of any intervention(s) are performed under the direction of the medical director of the hospital's infection control and prevention department.
Two interventions were made simultaneously to enhance the overall program: a molecular typing laboratory and a weekly planning meeting. The meeting included representatives from infection control, diagnostic medical microbiology (molecular epidemiology), pharmacy, and infectious diseases.
Observation Periods
The preintervention assessment for this evaluation began on September 1, 1992, the start of our 1993 fiscal year (FY). Data were collected and assessed by quarters for 2 years, through the fourth quarter, FY94 (June through August 1994). Initiating the weekly meetings and establishing the molecular typing laboratory occurred during the fourth quarter FY94; the laboratory was fully operational in the first quarter FY95. The intervention time was the first quarter FY95 through the fourth quarter FY99 (September 1994 through August 1999), the period when the enhanced program was in effect.
Organization of the Integrated Program
At the beginning of the intervention period, weekly meetings were initiated to review the ongoing short- and long-term trends in nosocomial infections within the center as well as activities of the infection control professionals and microbiology laboratory personnel; any needed changes were determined. The organizational structure for selecting microbes for typing was shared by the medical directors of infection control and clinical microbiology (12). During the study period, all VRE recovered from clinical and surveillance cultures were routinely genomically typed so that data were current within 2 weeks of an isolate's recovery. Periodic routine typing for surveillance of fluoroquinolone-resistant P. aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), Enterobacter cloacae, and Clostridium difficile was also done. Additional organisms for typing were selected by this working group through surveillance of microbiology culture reports discussed at the weekly meeting. The clinical microbiology laboratory referred organisms to the molecular typing section for analysis whenever requested to do so by this group.
Microbial Typing
Fingerprinting is done by extracting genomic DNA according to the technique of Pitcher et al., using the guanidium thiocyanate/EDTA/Sarkosyl (GES) reagent (17). Genomic DNA is digested with various enzymes according to the manufacturer's recommendation (GIBCO BRL, Gaithersburg, MD). Enzymes are selected based on published reports as well as ongoing experience within the typing section. When needed, two enzymes are used for typing to ensure the presence or absence of clonality. DNA fragments are separated into patterns by running them through an agarose gel with constant field electrophoresis. Usual run times are 16 to 24 hours, and the resultant gels are then stained with a nucleic acid bonding fluorescent agent, SYBR Green I (Molecular Probes; Eugene, OR), and visualized with UV illumination. Gels are imaged with a photo documentation system, Gel Print 2000i (Biophotonics; Ann Arbor, MI). The gels are photographed so that the molecular weight marker extends 6 cm to 7 cm in the image (the portion of the gel used for analysis [18]). Similarities between the new and reference types are scored by visual comparison of each 1-mm segment of the top 60 mm of the DNA band pattern. A similarity index is calculated from the number of identical 1-mm segments expressed as a percentage of the total number of 1-mm segments measured. More than six differences in the 1-mm segments constitute a similarity index of <90% and call for designation of a new type. Types are designated by letters, and a distinct band pattern within a type (similarity index >90%, but <100%) is designated by subscript Arabic numbers, indicating a subtype (e.g., A0, A1, A2). Subsequent organisms of the same genus and species are then compared with each main type or subtype to determine clonality. Organisms within the same type are considered related to each other for epidemiologic linkage.
Analysis of Cost Data
The hospital management engineering database was used to determine the total cost of inpatient care. Patient mix data were then used to determine the mean weighted cost per day for hospitalization within our center. The information used for cost calculations in this report is from 1999. The mean number of annual discharges was approximately 33,000 in 1995 to 39,000 in 1999, with a average of 36,444. We used the U.S. weighted mean of 4 days as the excess length of stay for a nosocomial infection in determining cost per patient (3). All other numbers in our calculations came directly from Northwestern Memorial Hospital data.
The resources needed for operating the molecular typing section were based on the cost of equipment, remodeling, reagent and other supplies, salaries and benefits for three technologists, plus all the institutional assessments (e.g., full-cost basis) required to operate a hospital laboratory. The nosocomial infection data in the two periods were analyzed by the Student t test (two-tailed distribution).
The initial impetus to develop our more integrated approach to infection control was VRE's emergence as a serious nosocomial problem. Use of molecular typing in an ongoing analysis of vancomycin-resistant Enterococcus faecium, the most important species in this epidemic, revealed that our persisting problem had evolved into a pattern of numerous "mini" patient-to-patient outbreaks of distinct clones rather than the spread of a single persisting strain (19). By assessing the VRE problem, we found that genomic typing could readily separate possible episodes of nosocomial infection spread into groupings of those that were likely, possibly, and unlikely due to patient-to-patient transmission (20). We could best use the typing capability to determine the probability of high microbial clonality (more than 90% of outbreak strains clonal), indicating patient-to-patient transmission; the probability of moderate clonality, suggestive of a nosocomial outbreak (35% to 75% clonality); or the probability of clonality with little evidence of horizontal spread (<20% clonality). Using this information, we determined what intervention was likely to control an apparent outbreak (20).
With a fully operational in-house typing facility, we were also able to use this resource to manage other nosocomial infections. During the last 2 years of this study, 25 possible microbial outbreaks were investigated by the typing laboratory, including VRE, fluoroquinolone-resistant P. aeruginosa, MRSA, E. cloacae, and C. difficile. A description of a few investigated episodes illustrates how we use the typing information.
Classic Spread of Nosocomial Infection
Nineteen isolates of vancomycin-resistant E. faecalis from 16 patients were detected in the microbiology laboratory in a 2-month period; isolates from 14 were from one of two clones (88%), indicating a high probability of nosocomial spread (14). Reviewing the origin of the culture requisitions in the microbiology laboratory did not indicate a possibility of close contact. However, an in-depth investigation found a direct connection between 11 of the 14 patients (14). Reinforcing infection control practices aborted the outbreak.
Moderate Likelihood of Spread of Nosocomial Infections
During a 1-month period, invasive infections caused by five isolates each of Klebsiella pneumoniae, S. epidermidis, and S. hemolyticus were detected in a special-care unit. DNA typing indicated 40% to 60% clonality for each of the bacterial species. This clustering was investigated, and patients with genetically identical organisms occupied adjacent beds. Erecting a barrier on the unit, along with educating medical staff, halted the spread of these infections (15).
Outbreaks not Caused by Patient-to-Patient Spread
Suspected outbreaks consisting of four isolates of K. pneumonia and 64 strains of Serratia marcescens were investigated in the ICUs of two hospitals. Both investigations showed 21% clonality, indicating unlikely patient-to-patient spread. Investigation suggested suboptimal handling of ventilator equipment, and both outbreaks were stopped by retraining of personnel using this equipment (12,15).
Pseudooutbreaks
Possible outbreaks occurred in the special-care nursery units of two hospitals, each of which had its own molecular typing section. One possible outbreak consisted of seven S. aureus strains, and the other of four isolates of gram-negative bacilli. Both sets of isolates were immediately typed and no (20%) clonality existed. No interventions were instituted, and the apparent outbreaks were determined to be normal variation in infections (15,21). Because of the rapid typing, one hospital avoided culture-based surveillance investigation of staff by the state department of health, and the other avoided closing the unit for a 30-day full disinfection and cleaning (done in previous suspected outbreaks).
Impact of Program Enhancements on Nosocomial Infections and Health-Care Cost
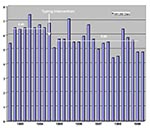
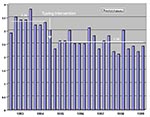
After molecular typing was added to our hospital infection control program, nosocomial infections decreased, as measured by the infection rate per 1,000 patient-days (Figure 1) and the proportion of patients with infections (Figure 2). The mean nosocomial infection rate fell from 6.49/1,000 patients days (standard deviation [SD] = ±0.66) in FY93-FY94 to 5.60/1,000 patient days (SD = ±0.74) in FY95-FY99 (p = 0.002). The percentage of patients with nosocomial infection dropped 23%, decreasing from 3.34% (SD = ±0.26) in the two preintervention years to 2.56% (SD = ±0.30) during the 5 years of our expanded program (p = 0.000006). The weighted cost of care per day in our hospital for FY95 was $1,650, and for FY99 it was $1,907. This increase was primarily due to steadily increasing severity of illness, largely from an increased volume of patients in our solid organ and bone marrow transplantation programs. The mean number of patients with nosocomial infections decreased by 283 per year, a reduction of more than 1,100 inpatient days. The costs avoided by using this calculation averaged more than $2,150,000/year, based on 1999 dollars.
The cost of this more integrated program was modest. Representatives from Infection Control, Infectious Diseases, Pharmacy, and Clinical Microbiology now meet together for 45 minutes each week to assess health-care associated infection problems and determine what needs to be done. For Microbiology, the equipment and remodeling cost for opening the typing laboratory totaled $180,050. By the fifth year, costs in the laboratory section were stable. The cost for the laboratory, includng three medical technologists, is $400,000 yearly. Virtually all these costs are borne by the hospital.
While we agree that new ways to assess infection control outcomes are needed (22), we chose two accepted measures and focused on our own hospital data that remained consistently assessed throughout the study. One measure was the nosocomial infection rate using 1,000 patient-days as the denominator. This rate compensated for any reduced length of stay and increased number of admissions during the observation period. During this period, the mean hospital length of stay dropped from 6.1 to 4.1 days, admissions increased from 31,000 to 39,000, total hospital days decreased from 190,000 to slightly more than 164,000, and overall severity of illness increased. The mean hospital-acquired infection rate during the preintervention period was 6.49/1,000 patient-days. In the first 2 years after the intervention, it had fallen to 5.79/1,000 patient days, and the overall 5-year intervention rate was 5.60/1,000 days, indicating the ability to maintain improved control of health-care associated infections over the long term. By contrast, the national average nosocomial infection rate per 1,000 patient days rose from 7.18 to 9.77 between 1975 and 1995, despite patient length of stay's falling from 7.9 to 5.3 days, and admissions declining from 37.7 million to 35.9 million (2). Our own rate has remained flat since our intervention period began, even though an increase (because our patients are more severely ill) might have been anticipated. This further suggests a continued positive outcome of the new integrated approach to our overall infection control program.
Our intrahospital comparison shows that before the enhanced approach was introduced, nosocomial infection developed in 3.3% of patients. In the 60 months after the practice change, health-care associated infections developed in 2.6% of admitted patients. More than 1,400 fewer patients acquired infections during this time, averting more than 50 expected deaths (23). Even with endemic vancomycin-resistant E. faecium, most of our outbreaks involve three or fewer patients (19).
While it is difficult to extrapolate beyond one's own medical center for an interhospital comparison (24), when our outcome is compared to what would be expected from the national average nosocomial infection rate of 4.4% to 5% of admitted patients in 1994 (23–25), and 1995 (2), the sustained rate reduction to <2.6% each year suggests that a predicted nosocomial infection was prevented in at least 2,600 patients during these 5 years at Northwestern Memorial Hospital as compared to the average 700-bed U.S. hospital.
Any of several molecular typing systems may be appropriate for determining microbial clonality, including restriction of genomic DNA with conventional electrophoresis (REA analysis), pulsed-field gel electrophoresis (PFGE), and rRNA gene probing (ribotyping). All methods are highly reproducible and have been applied to outbreaks. REA and PFGE have been shown equally effective for typing of VRE and C. difficile (20,26).
Typing of strains and assessment of clonality is usually available within 1 week of determining that an outbreak may exist and isolating suspected microbes. We have accomplished typing in as little as 48 hours. Identifying strains as clonal implies patient-to-patient spread and calls for enhanced infection control (barrier) precautions. Lack of clonality suggests other reasons for the apparent outbreak, such as antimicrobial-agent use pressure, failure of appropriate nursing-care practices, or simply random variation in the number of infections. Early knowledge of whether microbial clonality is present or absent focuses the scope of an investigation and facilitates appropriate intervention.
Even preventing asymptomatic colonization in health-care institutions is important since subsequent infection by virulent pathogens can have serious consequences (27). Our experience suggests that molecular typing technology can be very useful even when applied to a single medical center if it is part of a comprehensive infection control program.
Additional opportunities for use of molecular testing in detecting nosocomial multidrug-resistant pathogens will present themselves. Stosor et al. have demonstrated the capacity for rapid, sensitive detection of VRE contained in rectal swabs from colonized patients (28). These researchers reported that the cost of rapid detection using the polymerase chain reaction (PCR) was equal to one day of glove isolation, and that the PCR could be completed in a single 8-hour workday. As gene chip technology moves into clinical use, detecting a large number of resistance determinants soon after a patient is admitted to the hospital should be possible.
A microbiology laboratory fully equipped to cooperate in the management of nosocomial infections will also have the necessary infrastructure to act as a sentinel to detect new antimicrobial agent resistance, detect foodborne outbreaks of infection, and recognize and isolate pathogen(s) responsible for a bioterrorist attack. However, building such an infrastructure is not inexpensive and likely will not be undertaken by most hospitals when reimbursement for laboratory testing is declining. A system of incentives for hospitals to equip hospital-based microbiology laboratories with the needed tools is required. We suggest an approach that offers medical centers annual $300,000 to $500,000 federal grants to start a program of enhanced, comprehensive health-care infection control and prevention as described in this report. These grants could be administered through a federal program such as the Agency for Health-care Research and Quality or the Centers for Disease Control and Prevention, and monitored by current laboratory credentialing agencies such as the College of American Pathologists or the Joint Commission on Accreditation of Health-care Organizations. Rules for participation should be developed by professional societies with expertise in infection control and prevention. While such a grant program would cost up to $2 billion each year if all U.S. hospitals participated, the projected reduction in cost of treating nosocomial infections could reach over five times that amount. Monitoring compliance and outcome should be part of the annual grant renewal process. Such an approach is consistent with a recently released report delineating the federal response to reducing medical errors (29). Our data strongly suggest such an investment will not only reduce illness and death but also avert the high costs of treating avoidable infections.
Nearly 15 years ago, Haley et al. estimated that a 30% reduction in nosocomial infection would result in $300,000 of actual savings for each 250 beds in a single institution (30). The data from our 700-bed medical center substantiate their estimate, and the annual cost reduction of approximately $2 million is comparable to the $825,000 they estimated for our size institution, based on the mid-1980s dollars and health-care costs. Several years ago Lupski suggested the potential power of molecular epidemiology in assessing hospital outbreaks of nosocomial infection (31). He indicated that to gain acceptance, molecular methods need to be easy to perform; provide rapid, reliable information; give additional data not otherwise readily obtainable; and be cost-effective. Our experience has been that a highly integrated infection control program including a molecular typing section fulfills these criteria. The program currently in place, incorporating microbial genetic typing, is within the recently recommended infrastructure guidelines for essential activities of infection control and epidemiology in hospitals (32). Broadening such an approach for managing nosocomial infections to most U.S. hospitals is technically possible, medically useful, and economically justified.
Dr. Peterson directs the Clinical Microbiology Division as well as the Northwestern Prevention Epicenter at Northwestern Memorial Hospital. He is also professor of medicine and pathology at Northwestern University. His current research activities focus on using molecular testing methods to enhance infection control, understanding the molecular regulation of antimicrobial-agent resistance, designing new strategies for treating infections caused by reemerging bacteria, and developing new diagnostic tests for detecting microbial pathogens.
Dr. Noskin serves as the medical director of the Infection Control and Prevention Department at Northwestern Memorial Hospital as well as its health-care epidemiologist. He is an associate professor of medicine at Northwestern University Medical School as well as codirector of the Northwestern Memorial Hospital Infection Control and Prevention Project, a CDC-sponsored prevention epicenter.
Acknowledgments
The authors sincerely acknowledge the backing of the Northwestern Memorial Hospital leadership, particularly Larry Goldberg and Lawrence L. Michaelis, for providing exemplary support of an ongoing, comprehensive infection control and prevention program.
U.S. Public Health Service Grant no. UR8/CCU515081, the Excellence in Academic Medicine program from the state of Illinois, Northwestern Memorial Hospital, and Northwestern University supported this work.
References
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG. The nationwide nosocomial infection rate: a new need for vital statistics. Am J Epidemiol. 1985;121:159–65.PubMedGoogle Scholar
- Altman LK. Experts see need to control antibiotics and hospital infections. New York Times 1998 Mar 12.
- Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–42.PubMedGoogle Scholar
- O'Brien TF. The global epidemic nature of antimicrobial resistance and the need to monitor and manage it locally. Clin Infect Dis. 1997;24(Suppl 1):S2–8. DOIPubMedGoogle Scholar
- Peterson LR, and the ASCP susceptibility testing group. United States geographic bacteria susceptibility patterns. 1997. Diagn Microbiol Infect Dis. 1999;35:143–51.PubMedGoogle Scholar
- Bonten MJM, Hayden MK, Nathan C, van Voorhis J, Matushek M, Slaughter S, Epidemiology of colonization of patients and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–9. DOIPubMedGoogle Scholar
- Pfaller MA, Herwaldt LA. The clinical microbiology laboratory and infection control: emerging pathogens, antimicrobial resistance, and new technology. Clin Infect Dis. 1997;25:858–70. DOIPubMedGoogle Scholar
- Wilson MP, Spencer RC. Laboratory role in the management of hospital acquired infections. J Hosp Infect. 1999;42:1–6. DOIPubMedGoogle Scholar
- Fang FC, McClelland M, Guiney DG, Jackson MM, Hartstein AI, Morthland VH, Value of molecular epidemiologic analysis in a nosocomial methicillin-resistant Staphylococcus aureus outbreak. JAMA. 1993;270:1323–8. DOIPubMedGoogle Scholar
- Schiappa DA, Hayden MK, Matushek MG, Hashemi FN, Sullivan J, Smith KY, Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: A case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174:529–36. DOIPubMedGoogle Scholar
- Peterson LR, Petzel RA, Clabots CR, Fasching CE, Gerding DN. Medical technologists using molecular epidemiology as part of the infection control team. Diagn Microbiol Infect Dis. 1993;16:303–11. DOIPubMedGoogle Scholar
- Noskin GA, Lee J, Hacek DM, Postelnick M, Reisberg BE, Stosor V, Molecular typing for investigating an outbreak of Candida krusei. Diagn Microbiol Infect Dis. 1996;26:117–23. DOIPubMedGoogle Scholar
- Bodnar UR, Noskin GA, Suriano T, Cooper I, Reisberg BE, Peterson LR. Use of in-house molecular epidemiology and full species identification for controlling spread of vancomycin resistant Enterococcus faecalis isolates. J Clin Microbiol. 1996;34:2129–32.PubMedGoogle Scholar
- Hacek DM, Suriano T, Noskin GA, Kruszynski J, Reisberg B, Peterson LR. Medical and economic benefit of a comprehensive infection control program that includes routine determination of microbial clonality. Am J Clin Pathol. 1999;111:647–54.PubMedGoogle Scholar
- Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, National Nosocomial Infections Surveillance System (NNIS): Description of surveillance methods. Am J Infect Control. 1991;19:19–35. DOIPubMedGoogle Scholar
- Pitcher DG, Saunders NA, Owen RJ. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–6. DOIGoogle Scholar
- Clabots CR, Johnson S, Bettin KM, Mathie PA, Mulligan ME, Schaberg DR, Development of a rapid and efficient restriction endonuclease analysis (REA) typing system for Clostridium difficile and correlation with other typing systems. J Clin Microbiol. 1993;31:1870–5.PubMedGoogle Scholar
- Stosor V, Kruszynski J, Suriano T, Noskin GA, Peterson LR. Molecular epidemiology of vancomycin-resistant enterococci: a 2-year perspective. Infect Control Hosp Epidemiol. 1999;20:653–9. DOIPubMedGoogle Scholar
- Savor C, Pfaller MA, Kruszynski JA, Hollis RJ, Noskin GA, Peterson LR. Genomic methods for differentiating strains of Enterococcus faecium: an assessment using clinical epidemiologic data. J Clin Microbiol. 1998;36:3327–31.PubMedGoogle Scholar
- Keita-Perse O, Gaynes RP. Severity of illness scoring systems to adjust nosocomial infection rates: a review and commentary. Am J Infect Control. 1996;24:429–34. DOIPubMedGoogle Scholar
- Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552–7. DOIPubMedGoogle Scholar
- Archibald LK, Gaynes RP. Hospital acquired infections in the United States. Infect Dis Clin North Am. 1997;11:245–55. DOIPubMedGoogle Scholar
- Schifman RB, Howanitz PJ. Nosocomial infections. Arch Pathol Lab Med. 1994;118:115–9.PubMedGoogle Scholar
- Samore M, Killgore G, Johnson S, Goodman R, Shim J, Venkataraman L, Multicenter typing comparison of sporadic and outbreak Clostridium difficile isolates from geographically diverse hospitals. J Infect Dis. 1997;176:1233–8. DOIPubMedGoogle Scholar
- Noskin GA, Cooper I, Peterson LR. Vancomycin-resistant Enterococcus faecium sepsis following persistent colonization. Arch Intern Med. 1995;155:1445–7. DOIPubMedGoogle Scholar
- Stosor V, Tornatore MA, Noskin GA, Tenover FC, Peterson LR. Improved recovery of vancomycin-resistant enterococci (VRE) using a hot-start polymerase chain reaction (PCR) assay for the detection of vanA and vanB from rectal swabs.[Abstract C-366] In: Abstracts of the Ninety-eighth Annual Meeting of the American Society for Microbiology; 1998 May 17-21; Atlanta, GA. Chicago and Atlanta: Northwestern University and CDC; 1998.
- Shalala D, Herman A, Eisenberg J. Doing what counts for patient safety: Federal actions to reduce medical errors and their impact. Report of the Quality Interagency Coordination Task Force (QuIC). Washington: QuIC; 2000. p.1-95.
- Haley RW, White JW, Culver DH, Hughes JM. The financial incentive for hospitals to prevent nosocomial infections under the prospective payment system. JAMA. 1987;257:1611–4. DOIPubMedGoogle Scholar
- Lupski JR. Molecular epidemiology and its clinical application. JAMA. 1993;270:1363–4. DOIPubMedGoogle Scholar
- Scheckler WE, Brimhall D, Buck AS, Farr BM, Friedman C, Garibaldi RA, Requirements for infrastructure and essential activities of infection control and epidemiology in hospitals: a consensus panel report. Am J Infect Control. 1998;26:47–60. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 7, Number 2—April 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lance R. Peterson, Prevention Epicenter, Galter Carriage House, Suite 701, Northwestern Memorial Hospital, 251 East Huron Street, Chicago, IL 60611, USA; fax: 312-926-4139
Top