Volume 7, Number 5—October 2001
Synopsis
Cost-Effectiveness of a Potential Vaccine for Coccidioides immitis
Cite This Article
Citation for Media
Abstract
Coccidioidomycosis, a systemic fungal infection, affects Americans living in the Southwest. We evaluated the cost- effectiveness of a potential vaccine against Coccidioides immitis. Using a decision model we developed, we estimate that among children, vaccination would saved 1.9 quality-adjusted life days (QALD) and $33 per person. Among adults, screening followed by vaccination would save 0.5 QALD per person and cost $62,000 per quality adjusted life year gained over no vaccination. If the birth cohort in highly endemic counties of California and Arizona were immunized in 2001, 11 deaths would be averted and $3 million would be saved (in net present value) over the lifetime of these infants. Vaccination of adults to prevent disseminated coccidioidomycosis would provide a modest health benefit similar in magnitude to other vaccines but would increase net expenditures. Vaccination of children in highly endemic regions would provide a larger health benefit and would reduce total health care expenditures.
Coccidioides immitis, an infectious fungus, grows in the arid soil of the Central Valley of California, southern Arizona, and parts of Nevada, New Mexico, Utah, and Texas, as well as northern Mexico and parts of Central and South America. Regions endemic for C. immitis are home to approximately 20% of the U.S. population; an estimated 5 million persons live in the areas of highest endemicity (Figure 1) (1-3). Humans are infected by inhaling dust containing C. immitis arthroconidia. Dust storms (4,5) and activities associated with heavy dust exposure such as agricultural labor (6), excavating archeologic ruins (7), and military combat training (8,9) increase infection rates, total infectious load, and the proportion of symptomatic cases. Infection rates, as reflected by positive skin tests, have decreased since the 1940s and 1950s from as high as 8% per month to approximately 2% to 3% per year in highly endemic regions. This decrease is likely the result of reduced dust exposure attributable to lifestyle changes and urbanization. However, population growth in endemic regions has been steady and is projected to increase.
Recent epidemics in California and Arizona highlight the continuing public health threat and costs of coccidioidomycosis (10,11) and have led to efforts to develop a vaccine. An economic analysis of the 7,130 cases from 1991 to 1993 in California's Kern County demonstrated a cost to that county of $56 million (12). Because the U.S. training post for desert warfare is located in the Mojave Desert, an area endemic for C. immitis, a vaccine is a military as well as a civilian priority. A killed whole spherule vaccine that showed promise in animal trials was not well tolerated in humans (13). Current efforts of the Valley Fever Vaccine Project, a consortium of researchers funded primarily by the California Healthcare Foundation and the California Department of Health Services, focus on a number of antigens successful in mice models that may form the basis of a subunit vaccine (G. Rutherford, pers. comm.).
Despite the potential clinical value of a vaccine, the only study of the cost-effectiveness of a vaccination program was an Institute of Medicine (IOM) analysis for the purposes of setting priorities in vaccine development (14). The IOM concluded that vaccine development would cost more than $100,000 per quality-adjusted life year (QALY) gained, but included vaccine development costs and used a very simplified model. If the vaccine would not be cost-effective, current development efforts might be in vain. We present the results of a detailed cost-effectiveness analysis of a potential vaccine against C. immitis.
We used a decision model to evaluate the health and economic consequences of withholding the vaccine, screening and vaccinating only those susceptible to infection (screening/vaccination), or vaccinating all eligible persons. Taking a societal perspective, we calculated the incremental cost-effectiveness of these strategies (formula in Appendix), and we discounted both costs and benefits at an annual rate of 3%. We performed one-way sensitivity analyses on all the model variables, as well as a three-way sensitivity analysis of the most sensitive variables, and best- and worst-case scenarios.
We based the estimates for input variables on the literature whenever possible (Table A1). Our base-case estimates represent our judgment about the best estimate from the literature and discussion with experts. The ranges for costs represent variation by 25% above and below the base-case estimate, except where otherwise specified. The ranges for sensitivity analyses on quality-of-life estimates represent the 25th and 75th percentiles of patients' assessments of quality of life (15), except where otherwise specified. The authors had complete scientific and editorial independence from the funding agencies.
Decision Model
We developed a Markov model (Figure 2) with Decision Maker software (version beta 0.99.11.14.0a, 2000, S. Pauker, et al., Boston, MA) (16,17) to track hypothetical cohorts of patients who either did not receive the C. immitis vaccine, received it, or received it only if their skin test was negative. Patients who received the vaccine were at risk for pain at the injection site, mild to moderate fever, and anaphylaxis without death. Vaccinated patients were at decreased risk for extrapulmonary dissemination after primary infection. Patients neither immune from previous infection nor successfully vaccinated were at risk for C. immitis infection and serious sequelae. All patients were at risk for dying from other causes (18). We followed cohorts until death, with a Markov cycle length of 1 month.
Patient Population
We used epidemiologic and demographic data from two California counties (Kern and Tulare) and eight Arizona counties (Cochise, Gila, Graham, Maricopa, Pima, Pinal, Santa Cruz, and Yavapai) as proxies for the population features of highly endemic regions (2,19; Internal Revenue Service, unpub. data). We used two cohorts representing the weighted average age and prior probability of naturally acquired immunity among children (ages <17) and adults (ages 18 to 65) in 10 highly disease-endemic counties. New residents with no natural immunity were added to these cohorts. Children had an average age of 8.85 years, and 14.5% were naturally immune; adults had an average age of 39.51 years, and 47.5% were naturally immune.
Clinical Manifestations
Smith's classic studies in military recruits stationed in disease-endemic regions during World War II established that 60% of infected persons have no symptoms and 40% have a flulike illness (20). Asymptomatic infection and symptomatic infection with recovery confer probable lifelong immunity with a positive delayed-type hypersensitivity skin-test reaction to coccidioidin or spherulin. However, a fraction of persons do not have simple self-limited disease and instead more serious illness develops, such as respiratory failure, chronic pneumonia, and extrapulmonary dissemination (20-23). We present more clinical detail in Appendix I, online, and summarize probability estimates, costs, and utilities in the Table A1.
Vaccine and Skin-Test Characteristics
Animal trials of the vaccine rely on intraperitoneal or intranasal challenge with large loads of C. immitis. The outcome measure is survival or death from disseminated disease. Because the mechanism of action of the putative vaccine is not known, we conservatively assumed that vaccination prevents extrapulmonary dissemination but not primary infection. We evaluated this assumption in a sensitivity analysis.
We assumed that all vaccinated persons comply with 3 doses in 1 year and all develop an antibody response. We assumed that the vaccine reduced the probability of dissemination by 75%, with lifelong duration, based on experience with hepatitis B vaccine, a widely used subunit vaccine. We examined the effects of compliance with each strategy and the effect of waning immunity.
Using data from hepatitis B vaccine as a proxy, we assumed that 25% of patients would experience mild side effects such as local arm pain, fever, and nausea and that 1 in 600,000 patients would experience nonfatal anaphylaxis requiring hospitalization (24).
For screening before vaccination, studies suggested a sensitivity of 70% and a specificity of 90% for the spherulin intradermal test (25-30).
Quality of Life
Our model included intermediate health states that may be associated with decrements in quality of life. To adjust for such decrements, we included quality adjustments in our model (Table A1). Because there are no quality-of-life studies for coccidioidomycosis, we used proxy health state utilities in published studies or clinical judgment. All illness utilities were multiplied by age-specific "healthy" utilities to account for the age at which an illness was contracted. We assumed that primary pulmonary infection causes a substantial heath status decrement only in those who are sick enough to be diagnosed. Among those with primary pulmonary disease that goes undiagnosed, we assigned a health state utility equal to well for this illness episode, but accounted for lost time from work due to illness (see Costs, below). For the short-term vaccine side effects, we capture quality-of-life impact as a decrease in utility of one-tenth of a quality-adjusted day. The base case and ranges for these quality-of-life adjustments reflect our judgment about the impact of these episodes on patients. We evaluated the effects of these utility assumptions in sensitivity analyses.
Costs
Direct Medical Costs
All costs are presented in 2000 U.S. dollars (Table A1). For costs of inpatient care, we used cost-adjusted charges from the 1996 Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project (31). For outpatient services, we used the 2000 Medicare national physician fee schedule (32). For outpatient services not listed in the Medicare fee schedule, we used reimbursement received by Kern County service providers (R. Talbot, pers. comm.) We assumed that patients severely disabled by coccidioidal meningitis would require home support and used the average payment per Medicare home health beneficiary as a proxy for this cost (33).
Vaccine and Other Costs
In our base-case analysis, we assumed the cost of the vaccine was $180. We chose this value for the base case because it is the reimbursed fee for the three-shot hepatitis B series, an existing subunit vaccine. We examined a broad range of vaccine costs ($100 to $400) in sensitivity analyses.
For circumstances in which quality-of-life changes do not capture the inconvenience of illness or medical care, we included time costs, as noted in the Appendix.
Prevention of Illness and Death
We calculated the number of cases of disseminated disease and deaths per 100,000 persons that would be prevented over the lifetime of each cohort if children and adults were immunized, assuming rates of vaccine coverage of 40%, 60%, 80%, and 100% (Table 1). For example, if 60% of children were vaccinated, 93 cases of dissemination, 64 cases of disability, and 7 deaths would be averted and $2 million saved per 100,000 population. If 60% of adults underwent screening followed by vaccination, 38 cases of dissemination, 24 cases of disability, and 2 deaths would be prevented at a cost of $5.4 million per 100,000 population.
Costs and Effectiveness
We calculated the effectiveness, costs, and cost-effectiveness of each strategy in each population, expressed in Table 2 as the per-person cost and health benefit. Among children, vaccination saved 1.9 quality-adjusted life days (QALD) and $33 per person. Among adults, screening followed by vaccination saved 0.5 QALD per person and cost $62,000 per QALY gained over no vaccination. For adults, the incremental gain from vaccinating all persons compared with screening followed by vaccination contributed an additional 0.05 QALD at a cost of $235,000 per QALY gained.
Sensitivity Analyses
We performed one-way sensitivity analyses over the ranges of all input variables listed in the table in Appendix II and on critical assumptions, such as the vaccine mechanism of action and target population. Among children, vaccination was no longer cost-saving at the lowest ranges of vaccine efficacy, infection rate, dissemination rate, long-term care cost for severe disability, and medical follow-up cost after nonmeningeal dissemination and chronic pulmonary disease; the vaccine also was not cost-saving in children when we used the highest ranges of vaccine cost, meningitis mortality, emigration, and discount rate among children. The counter-intuitive effect of meningitis deaths is due to the decrease in survivors subject to long-term care costs from post-meningitis disability. Among adults, sensitivity analyses that changed the cost-effectiveness ratio by >$40,000 per QALY gained included infection rate, vaccine effectiveness, discount rate, vaccine cost, dissemination rate, emigration rate, and office visit time. Only the vaccine cost changed the preferred strategy among adults. Below $106 per 3 doses, vaccination was preferred over screening/vaccination, with a cost-effectiveness ratio of $30,000 per QALY gained.
Vaccine Duration
Our base-case analysis assumed lifetime immunity after vaccination, but vaccine protection may wane. If vaccine protection waned to zero in 15 years, vaccination saved 0.82 QALD per child and cost $47,300 per QALY gained, and screening/vacccination saved 0.28 QALD per adult and cost $165,500 per QALY gained.
Vaccine Mechanism of Action
If the vaccine prevented primary infection rather than dissemination alone, vaccination saved 2.26 QALD and $46 per vaccinated child over no vaccination. Screening/vaccination saved 0.66 QALD per adult, costing $46,500 per QALY saved.
Three-Way Sensitivity Analysis of Infection Rate, Vaccine Effectiveness, and Cost
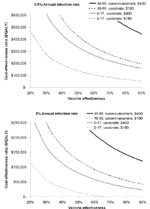
Our base case assumed an infection rate of 2% per year, vaccine effectiveness of 75%, and a vaccine cost of $180. However, the vaccine may have some use in areas of lower endemicity. Also, because the vaccine does not yet exist, its cost and effectiveness are unknown. We present the effects of varying costs and effectiveness of the vaccine under two conditions: 0.5% infection rate per year and 2% per year (our base case of highly endemic regions) (Figure 3). In our base case for children, the $180 vaccine remains cost-saving down to an effectiveness of 65% and costs <$50,000 per QALY until vaccine effectiveness drops below 30%, confirming that the vaccine need not be highly effective to be cost-effective in younger populations. A $400 vaccine costs approximately $50,000 per QALY gained among children at 65% effectiveness. A $400 vaccine would be economically unfavorable for the screening/vaccination strategy in adults at an annual infection rate of 2%, even at 90% vaccine effectiveness. At an infection rate of 0.5% per year, as might be seen in southern California (34), all strategies except $180 vaccination at 90% effectiveness in children are economically unfavorable.
Discount Rate
Among children, vaccination saved 5.95 QALD and $563 over no vaccination when costs and benefits were not discounted. At a discount rate of 5%, vaccination was no longer cost-saving at $27,600 per QALY gained. For adults, changing the discount rate from 0% to 5% changed the cost-effectiveness ratio of screening/vaccination compared with no vaccination from $30,800 to $155,000 per QALY gained.
Dissemination Rate
Our base-case analysis used a dissemination rate of 0.38% based on the assumption that only blacks and Asians had higher rates of dissemination than whites. We evaluated a range of 0.25% to 0.55% to determine the effect of assuming all non-whites (including Hispanics) had rates of dissemination equal to that of whites (0.25%) or that of blacks (3.4%). Among children, vaccination saved 2.71 QALD and $143 per child vaccinated at an overall dissemination rate of 0.55%, and 1.22 QALD at a cost of $15,300 per QALY gained at the 0.25% dissemination rate. Among adults, varying the dissemination rate from 0.25% to 0.55% changed the cost-effectiveness ratio of screening/vaccination over no vaccination from $25,400 to $125,000 per QALY gained.
New Residents
If one considers new residents separately, vaccination saved 2.18 QALD and $75 per immigrant child vaccinated, and saved 1.13 QALD per immigrant adult vaccinated, costing $13,500 per QALY gained.
We present best- and worst-case scenarios in Appendix.
We evaluated the usefulness of a potential vaccine against C. immitis in highly disease-endemic regions. Vaccination was cost-saving among children. A screening/vaccination strategy cost $62,000 per QALY gained among adults. Although the increase in quality-adjusted life expectancy from an immunization program is modest for one individual patient, this aggregates to an important number of illnesses and deaths prevented (Table 1). Furthermore, the life expectancy gains are comparable with gains from other immunizations. Vaccination for coccidioidomycosis (universally among children and after screening in adults) saved 0.14 to 0.47 life days and 0.53 to 1.86 QALD per person over no vaccination. In comparison, vaccination against pneumococcal bacteremia among elderly people saves 1.2 QALD per person vaccinated (35); infant vaccinations against measles, mumps, rubella, and pertussis each save 2.7, 3, 0.3, and 3.3 life days, respectively (36). To further place benefits in perspective, if the birth cohort in highly endemic counties of California and Arizona were immunized in 2001, 11 deaths would be averted and $3 million would be saved (in net present value) over the lifetime of these children.
The only previous analysis of the cost-effectiveness of a vaccine against coccidioidomycosis drew different conclusions. An IOM report considered the costs and benefits of research and development into a vaccine for C. immitis and found that the immunization of infants in endemic regions and immigrants of any age would cost >$100,000 per QALY (14). Our analysis evaluated a different question: If a vaccine were currently available, would it be cost-effective to immunize people in highly endemic regions? Although the IOM report acknowledged that the committee used rough estimates and a simplified model, the primary reason that it reached a different conclusion was that the cost of vaccine development was included in the IOM model. Vaccine development costs will influence the sale price of the vaccine. Project directors of the Valley Fever Vaccine Project estimate that research and development through phase III clinical trials, supported largely by public and private philanthropic sources, will cost $28 million (G. Rutherford, pers. comm.). This figure is 10 times lower than that used by the IOM in its analysis. To recoup $28 million over 5 years by immunizing 60% of the 90,000 annual birth cohort in highly endemic regions of California and Arizona, the vaccine would have to be priced at $100 per vaccinated person. This is less than the $180 vaccine cost assumed in our base-case analysis.
Sensitivity analyses found that the vaccine would be cost-effective in children under assumptions of waning immunity and would be even more effective and cost-saving in children and cost-effective in adults if the vaccine prevented primary infection. In highly endemic regions, vaccination of children is cost-effective even at relatively low levels of vaccine effectiveness if the vaccine is priced at $180. A $180 vaccine had to be >85% effective for screening/vaccination to cost $50,000 per QALY in adults. Paradoxically, an even higher annual infection rate would make a screening/vaccination strategy less favorable in adults because most of them would have already acquired natural immunity by age 40. At an annual infection rate one-fourth of that seen in the Central Valley of California, such as might be seen in southern California, the only cost-effective strategy would be to immunize children with an inexpensive and highly efficacious vaccine. If dissemination rates are higher than our base case, as might be seen in the elderly and those with chronic diseases, screening/vacccination becomes cost-effective for adults.
The vaccine is much more effective in persons without naturally acquired immunity. A vaccination strategy that targeted new residents in highly endemic regions would exploit easily obtainable risk factor information. However, such a policy might be unacceptable to members of the population excluded by such a strategy. Finally, because vaccination is a preventive intervention that can take years before accruing a health benefit (in contrast to acute health-care interventions), our results were highly sensitive to discount rate.
Our model has limitations. The ecology of endemic regions has changed substantially with urbanization since Smith's study in the 1940s (20). Furthermore, his studies documenting infection and dissemination were conducted in the military, which may not be representative of general population exposures. Our base-case analysis used the demographics in highly endemic areas to estimate a composite dissemination rate for a multiracial population. We did not model race independently, nor did we model exposure risk. Workers with high levels of dust exposure may have higher rates of infection and more to gain from early immunization.
Our model assumed that all age groups without prior immunity were at equal risk for adverse health outcomes and had identical health-care costs. However, illness severity and costs may be higher among the elderly. Reported cases of coccidioidomycosis during Arizona's 1991 to 1995 epidemic occurred disproportionately among older adults (11). The study did not discern whether this was due to reporting differences, immune-suppressing coexisting conditions, a preponderance of new immigrants with higher risk for infection, or an independent age-related phenomenon. The death rate from primary pulmonary infection is as high as 26.8% among persons over 65 (37), much higher than our composite base-case estimate of 0.5%. The Kern County data on which we based our cost assumptions revealed that hospitalization, the greatest health-care expenditure associated with acute illness, was 4.2 times more likely among people >50 years old. Thus, we may have underestimated the potential effectiveness and cost savings of the vaccine among older adults.
Finally, our analysis did not model the effects of an immunization program in immunocompromised patients. Persons with compromised cell-mediated immunity, including those with AIDS, malignancy, or therapeutic medical immunosuppression, have higher rates of serious illness. Many of these patients may have reactivation of previous infection; it is unclear whether vaccination would be useful in this clinical scenario. This is an area for future research as results from clinical trials become available.
An uncommon disease in a national context, coccidioidomycosis has substantial ramifications for several regions where epidemics have produced reported annual case rates as high as 15 per 100,000 and substantial economic impact. Diseases affecting <200,000 persons annually (orphan diseases) require more incentives for research and development. Our findings may contribute to the policy decisions for vaccine development and distribution for C. immitis (39). Our analysis suggests that a vaccine against C. immitis would have substantial public health benefit. An update of this cost-effectiveness analysis can be performed when the results of human vaccine trials become available.
Clinical and Economic Background
We present here detailed information to supplement the methods and results sections of the manuscript.
Cost-Effectiveness Ratio Formula
The incremental cost-effectiveness ratio is calculated as:
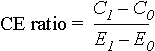
where the subscript 1 denotes the intervention under study and subscript 0 the alternative with which it is compared, C1 and C0 are the net present values of costs accrued when the intervention and its alternative are used, and E1 and E0 are their respective health outcomes (39). Thus, C1 - C0 is the incremental cost and E1 - E0 is the incremental effectiveness of the intervention over the alternative.
Population Characteristics
In the 10 highly coccidioidomycosis-endemic California counties used for our analysis, the annual birth cohort is 90,000 per year and the net annual population growth ranges from 0.3% in Cochise to 3.6% in Pinal (40). Using age-grouped U.S. census data and regional skin test surveys, we calculated a weighted population mean age and mean prior probability of immunity for children (<17: age 8.85, prior 14.5%) and adults (18-65: age 39.51, prior 47.5%). These figures account for population inflow of nonimmune persons from nonendemic regions. Inflow and outflow migration data at the county level are available from the Internal Revenue Service (IRS, unpub. data). For these 10 counties, there is an annual inflow of 5.4% from, and outflow of 4.2% to, nonendemic regions. No data source provides person-level migration data. To account for vaccinees' accruing cost without benefit, we assumed that 0.5% of yet uninfected persons move from highly disease-endemic regions to nonendemic regions each year, corresponding to one third of a birth cohort emigrating by age 65 years.
We also used census data to calculate the proportion of whites, blacks, Hispanics, native Americans, and Asians in these 10 counties. We used this information to calculate a composite dissemination rate (see "Disseminated Infection," below).
Clinical Manifestations of Coccidioidomycosis
Pulmonary infection
The initial respiratory infection with Coccidioides immitis, if symptomatic, is a 2- to 3-week flulike respiratory ailment with cough, fever, and chest pain that may be associated with erythema nodosum. The illness is usually self-limited. If symptoms last longer than 6 weeks, the illness is considered a chronic pulmonary infection (41-45). Among the 40% of infected persons who have symptomatic primary pulmonary infection, from one third to two thirds visit their physicians (46,47), and 1 in 10 patients is serologically diagnosed (John Galgiani, pers. comm., 1999). Because case series in published articles include only patients with diagnosed coccidioidomycosis, we built the probability of diagnosis proximally into the disease model. A proportion of cases progress to respiratory compromise requiring hospitalization; 42.5% of the hospitalizations for coccidioidomycosis in Arizona during 1995 were due to primary pulmonary infection and 11.4% to chronic pulmonary infection (48). The overall death rate from serious pulmonary disease is 0.5% (49). Using Kern County utilization data, we assume that 27% of diagnosed primary pulmonary cases are hospitalized and that each case has an average of 6 physician visits (50).
Disseminated Infection
A small fraction of infections disseminate. We calculated the overall dissemination rate in highly disease-endemic areas by using black and white dissemination rates observed among the military (46) and 1999 U.S. census data for population by race in the 10 counties used in our analysis (40). Higher rates of dissemination occur among blacks, Filipinos, and Native Americans (46,51-63). It is unclear whether race is a biologic risk factor via human lymphocyte antigen haplotypes (64) or a confounding variable for those in high-risk occupations and poorer housing conditions that may increase exposure to C. immitis (62,65; Rosenstein N, unpub. data). Even a seemingly uniform exposure, such as occurred in a dust storm carrying arthroconidia from the Central Valley into urban Sacramento in 1979 (55,66), may not be uniform if different groups have different housing quality (e.g., air-conditioning versus open windows and fans). We assumed that whites and Hispanics had a rate of 0.25% (the rate among whites in Smith's studies [66]) and that blacks, Native Americans, and Asians had a rate of 3.4% (the rate among blacks in Smith's studies), for an overall rate of 0.38%. In our model, we took advantage of the Kern County finding that 4.7% of all diagnosed cases of primary pulmonary disease disseminated (67). Folding this number back into the overall dissemination rate of 0.38%, we calculated that nondiagnosed primary pulmonary infection and asymptomatic cases disseminated at a rate of 0.20%. We did not model the increased risk of dissemination that may accompany pregnancy (65,68-74).
A utilization study from Kern County found that 84% of all disseminated cases were hospitalized and that each patient had an average of 35 visits in the course of one year (50). We assumed that all meningitis patients were hospitalized, leaving 4/14 non-meningeal dissemination cases to be managed without hospitalization. Further, we assumed that most physician visits took place during the incident month and that follow-up care involved one visit per month. Costs for treating incident disseminated disease, which may include the use of amphotericin B, are contained in the cost of hospitalization for incident disease. We assumed all meningitis patients were treated chronically with 800 mg of fluconazole and that nonmeningeal dissemination patients were treated with either 400 mg of fluconazole or 400 mg of ketoconazole.
Disability
Some patients with disseminated coccidioidomycosis become disabled, causing a lower quality of life and higher costs for long-term care. No studies were published on this topic. Experts assert that half of patients who survive C. immitis meningitis suffer moderate disability, such as chronic cluster headaches, poor concentration, low back pain, and residual focal neurologic defects; and that one sixth of patients suffer disability severe enough to require skilled nursing or home-care support (e.g., major neurologic deficits and coma). One third of patients who survive nonmeningeal dissemination have an orthopedic disability (John Galgiani, pers. comm., 2000).
Costs
Inpatient costs used discharge diagnoses of primary pulmonary coccidioidomycosis (ICD-9 114.0, Diagnosis-Related Group [DRG] 80), coccidioidal meningitis (ICD-9 114.2, DRG 20), disseminated coccidioidomycosis (ICD-9 114.3, DRG 423), and anaphylaxis (ICD-9 999.4, DRG 447) (75). Outpatient costs from the National Medicare Fee Schedule included intermediate established outpatient office visit (Current Procedural Terminology [CPT] code 99212 and coccidoidomycosis skin testing (CPT code 86490). Other costs obtained from the Kern County Public Health Department included the C. immitis laboratory tests: enzyme immunoassay (EIA) immunoglobulin (Ig) M (CPT code 83171), complement fixation (CPT code 86171), and immunodiffusion (CPT code 86331) and hepatitis B immunization (CPT code 90746).
By calculating the weighted average adjusted gross income for the 10 California and Arizona counties in our model from IRS tax return data (IRS, unpub. data), we obtained a figure of $12/hour as the value of a person's time. For a person with undiagnosed primary pulmonary illness, we assumed 5 days of lost time from work for a working adult (<65 years old) and 3 days of lost time from work for the parent of a sick child (children, on average, have less severe primary illness). We assumed that the first vaccination in a vaccination strategy would be delivered during a visit scheduled for another purpose, adding 15 minutes to the visit. The two successive vaccinations would require clinic visits with 1-hour travel and waiting time and 15 minutes vaccination time. For a screen and vaccinate strategy, the screening visit and the successive reading plus vaccination (if indicated) would take 1 hour 15 minutes, as would each of two successive vaccination visits.
Results: Best- and Worst-Case Scenarios
We examined scenarios in which the input variables were most and least favorable to vaccination. Under a best-case scenario, in which the vaccine prevents infection, childhood vaccination saved $608 and 112 quality-adjusted life days (QALD) per person, and a screen and vaccinate strategy saved $55 and 16 QALD per adult over no vaccination. A worst-case scenario, in which the vaccine is ineffective, caustic, costly, and applied to a population in a lightly disease-endemic region, the vaccine does more harm than good in all ages. The probability of either scenario, however, is essentially zero.
Dr. Owens was supported by a Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service. Dr. Barnato was supported by a training grant from the Agency for Healthcare Research and Quality. This work was supported in part by a contract from the California State University Bakersfield Foundation.
Dr. Barnato was a fellow in health care research and policy in the Department of Medicine at Stanford University School of Medicine when she completed this work. She is currently assistant professor of medicine at the University of Pittsburgh School of Medicine. Her research interests include quantitative assessment of preventive interventions and policy tools for quality improvement and cost control in health care.
Acknowledgment
The authors thank George Rutherford, John Galgiani, Royce Johnson, Hans Einstein, John Caldwell, and David Stevens for their expert advice; Janet Morrison and Kristen Jacobs for their library research assistance; and Todd Wagner for his cost- accounting assistance.
References
- Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis. 1996;2:192–9. DOIPubMedGoogle Scholar
- Maddy KT. The geographic distribution of Coccidioides immitis and possible ecologic implications. Ariz Med. 1958;15:178.PubMedGoogle Scholar
- Population estimates. Washington: U.S. Census Bureau; 1999.
- Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E, An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301:358–61. DOIPubMedGoogle Scholar
- Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA, A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997;277:904–8. DOIPubMedGoogle Scholar
- Johnson WM. Occupational factors in coccidioidomycosis. J Occup Med. 1981;23:367–74.PubMedGoogle Scholar
- Werner SB, Pappagianis D, Heindl I, Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med. 1972;286:507–12. DOIPubMedGoogle Scholar
- Standaert SM, Schaffner W, Galgiani JN, Pinner RW, Kaufman L, Durry E, Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J Infect Dis. 1995;171:1672–5. DOIPubMedGoogle Scholar
- Hooper R, Poppell G, Curley R, Husted S, Schillaci R. Coccidioidomycosis among military personnel in Southern California. Mil Med. 1980;145:620–3.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Coccidioidomycosis--California, 1991-1993. MMWR Morb Mortal Wkly Rep. 1994;43:421–3.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Coccidioidomycosis--Arizona, 1990-1995. MMWR Morb Mortal Wkly Rep. 1996;45:1069–73.PubMedGoogle Scholar
- Caldwell J, Welch G, Johnson RH, Einstein HE. The economic impact of coccidioidomycosis in Kern County, California, 1991 to 1993. In: Einstein HE, Catanzaro A, editors. Coccidioidomycosis: proceedings of the 5th international conference. Washington: National Foundation for Infectious Diseases; 1994: p. 88-97.
- Pappagianis D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am Rev Respir Dis. 1993;148:656–60. DOIPubMedGoogle Scholar
- Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st century: a tool for decision making. Washington: Division of Health Promotion and Disease Prevention, Institute of Medicine; 1999.
- Gold M, Franks P, McCoy K, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–92. DOIPubMedGoogle Scholar
- Beck JR, Sauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–58. DOIPubMedGoogle Scholar
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention, National Center for Health Statistics (NCHS). Monthly vital statistics report: annual summary of births, marriages, divorces, and deaths: United States. Hyattsville (MD): NCHS; 1995.
- Centers for Disease Control and Prevention. Coccidioidomycosis--United States, 1991-1992. MMWR Morb Mortal Wkly Rep. 1993;42:21–4.PubMedGoogle Scholar
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of diseases. Am J Public Health. 1946;36:1394–402. DOIGoogle Scholar
- Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin Infect Dis. 1993;16:349–54. DOIPubMedGoogle Scholar
- Arsura E, Caldwell J, Johnson R, Einstein H, Welch G, Talbot R, Coccidioidomycosis epidemic of 1991: epidemiologic features. In: Einstein HE, Catanzaro A, editors. 5th International Conference on Coccidioidomycosis. Stanford University, Aug 24-27, 1994. Bethesda (MD): National Foundation for Infectious Diseases; 1996. p. 98-107.
- Galgiani JN. Coccidioidomycosis: a regional disease of national importance; rethinking approaches for control. Ann Intern Med. 1999;130:293–300.PubMedGoogle Scholar
- Assad S. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18:57–67. DOIPubMedGoogle Scholar
- Lebowitz MD, Johnson WM, Kaltenborn W. Coccidioidin skin test reactivity and cross-reactivity with histoplasmin in a Tucson population. In: Ajello L, editor. Coccidioidomycosis: current clinical and diagnostic status. Vol 1. Miami: Symposia Specialists; 1977. p. 45-61.
- Dodge RR, Lebowitz MD, Barbee R, Burrows B. Estimates of C. immitis infection by skin test reactivity in an endemic community. Am J Public Health. 1985;75:863–5. DOIPubMedGoogle Scholar
- Geller RD, Maynard JE, Jones V. Coccidioidin sensitivity among Southwestern American Indians. Am Rev Respir Dis. 1973;107:301–2.PubMedGoogle Scholar
- Stevens DA, Levine HB, Deresinski SC, Ten Eyck DR, Restrepo MA. Epidemiological and clinical skin testing studies with spherulin. In: Ajello L, editor. Coccidioidomycosis: current clinical and diagnostic status. Vol 1. Miami: Symposia Specialists; 1977. p. 107-14.
- Levine HB, Gonzalez-Ochoa A, Ten Eyck DR. Dermal sensitivity to Coccidioides immitis. a comparison of responses elicited in man by spherulin and coccidioidin. Am Rev Respir Dis. 1973;107:379–86.PubMedGoogle Scholar
- Levine HB, Restrepon A, Eyck DR, Stevens DA. Spherulin and coccidioidin: cross-reactions in dermal sensitivity to histoplasmin and paracoccidioidin. Am J Epidemiol. 1975;101:512–6.PubMedGoogle Scholar
- Healthcare Cost and Utilization Project: Nationwide Inpatient Sample. Rockville (MD): Agency for Healthcare Research and Quality; 1996.
- Revised 2000 National physician fee schedule. Baltimore: Health Care Financing Administration; 2000.
- Manton KG, Etallard E. Chapter 6: Program payment and utilization trends for Medicare beneficiaries with disabilities. In: Wiener JM, Clauser SB, Kenner DL, editors. Persons with disabilities. Washington: The Brookings Institution; 1995. p. 117-62.
- Klotz AL, Biddle M. Coccidioidin skin test survey of San Fernando Valley State college students over a five year period. In: Ajello L, editor. The Second Symposium on Coccidioidomycosis. Phoenix (AZ): University of Arizona Press; 1965. p. 251-3.
- Sisk JE, Moskowitz AJ, Whang W, Lin JD, Fedson DS, McBean AM, Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA. 1997;278:1333–9. DOIPubMedGoogle Scholar
- Wright JC, Weinstein MC. Gains in life expectancy from medical interventions--standardizing data on outcomes. N Engl J Med. 1998;339:380–6. DOIPubMedGoogle Scholar
- Arsura EL. The association of age and mortality in coccidioidomycosis [letter]. J Am Geriatr Soc. 1997;45:532–3.PubMedGoogle Scholar
- Lang J, Wood SW. Development of orphan vaccines: an industry perspective. Emerg Infect Dis. 1999;5:749–56. DOIPubMedGoogle Scholar
- Garber AM. Advances in cost-effectiveness analysis of health interventions. In: Culyer A, Newhouse J, editors. Handbook of health economics. Vol. 1. New York: Elsevier Science B; 2000. p. 182-219.
- Population estimates. Washington: U.S. Census Bureau; 1999.
- Sarosi GA, Parker JD, Doto IL, Tosh FE. Chronic pulmonary coccidioidomycosis. N Engl J Med. 1970;283:325–9. DOIPubMedGoogle Scholar
- Bayer AS. Fungal pneumonias: pulmonary coccidioidal syndromes (Part 2). Miliary, nodular, and cavitary pulmonary coccidioidomycosis; chemotherapeutic and surgical considerations. Chest. 1981;79:686–91. DOIPubMedGoogle Scholar
- Bayer AS. Fungal pneumonias; pulmonary coccidioidal syndromes (Part 1). Primary and progressive primary coccidioidal pneumonias--diagnostic, therapeutic, and prognostic considerations. Chest. 1981;79:575–83. DOIPubMedGoogle Scholar
- Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin Infect Dis. 1993;16:349–54. DOIPubMedGoogle Scholar
- Galgiani JN. Coccidioidomycosis. In: Remington JS, Swartz MN, editors. Curr Clin Top Infect Dis 1997:188-204.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of diseases. Am J Public Health. 1946;36:1394–402. DOIGoogle Scholar
- Smith CE. Coccidioidomycosis. In: Hayes SB, Coate JB, editors. Preventive medicine in World War II. Vol. 4. Washington: Department of the Army; 1958. p. 285.
- Centers for Disease Control and Prevention. Coccidioidomycosis--Arizona, 1990-1995. MMWR Morb Mortal Wkly Rep. 1996;45:1069–73.PubMedGoogle Scholar
- Arsura E, Caldwell J, Johnson R, Einstsein H, Welch G, Talbot R, Coccidioidomycosis epidemic of 1991: epidemiologic features. In: Einstein HE, Catanzaro A, editors. 5th International Conference on Coccidioidomycosis. Stanford (CA): National Foundation for Infectious Diseases; 1994. p. 98-107.
- Caldwell J, Welch G, Johnson RH, Einstein HE. The economic impact of coccidioidomycosis in Kern County, California, 1991 to 1993. In: Einstein HE, Catanzaro A, editors. Coccidioidomycosis: Proceedings of the 5th International Conference. Washington: National Foundation for Infectious Diseases; 1994. p. 88-97.
- Gifford M, Buss W, Douds R. Annual Report of the Kern County Health Department for the fiscal year July 1, 1936 to June 30, 1937. Bakersfield (CA): The Department; 1937.
- Willett F, Weiss A. Coccidioidomycosis in Southern California: Report of a new endemic area with a review of 100 cases. Ann Intern Med. 1945;23:349–75.
- McCracken BE. (Surgeon's Office 6th Army). Final report of coccidioidomycosis research project at Camp Roberts, California, 1 Sept 1952-15 Oct 1953.
- Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129:527–30.PubMedGoogle Scholar
- Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E, An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301:358–61. DOIPubMedGoogle Scholar
- Cody S, Emergy K, Werner SB, Talbot R, Hajjeh R, Reingold A, Population-based surveillance for coccidioidomycosis in Kern County, CA, 1995 [poster #P-16.2]. International Conference on Emerging Infectious Diseases. Atlanta (GA), Mar 8-11, 1998.
- Pappagianis D, Lindsay S, Beall S, Williams P. Ethnic background and the clinical course of coccidioidomycosis [letter]. Am Rev Respir Dis. 1979;120:959–61.PubMedGoogle Scholar
- Sievers ML. Disease patterns among southwestern Indians. Public Health Rep. 1966;81:1075–83. DOIPubMedGoogle Scholar
- Sievers ML. Disseminated coccidioidomycosis among southwestern American Indians. Am Rev Respir Dis. 1974;109:602–12.PubMedGoogle Scholar
- Sievers ML. Prognostic factors in disseminated coccidioidomycosis among Southwestern American Indians. In: Ajello L, editor. Coccidioidomycosis: Current clinical and dignostic satus. Vol. 1. Miami: Symposia Specialists; 1977. p. 63-78.
- Sievers M. Racial susceptibility to coccidioidomycosis [letter]. N Engl J Med. 1980;302:58–9. DOIPubMedGoogle Scholar
- Sievers ML, Fisher JR. Decreasing incidence of disseminated coccidioidomycosis among Piman and San Carlos Apache Indians. A probable environmental basis. Chest. 1982;82:455–60. DOIPubMedGoogle Scholar
- Sievers ML, Nelson RG, Bennett PH. Adverse mortality experience of a Southwestern American Indian community: overall death rates and underlying causes of death in Pima Indians. J Clin Epidemiol. 1990;43:1231–42. DOIPubMedGoogle Scholar
- Louie L, Ng S, Hajjeh R, Johnson R, Vujia D, Werner SB, Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672–80. DOIPubMedGoogle Scholar
- Jinadu BA. Valley Fever task force: report on the control of Coccidioides immitis. Bakersfield (CA): Kern County Health Department; Aug 1995.
- Drutz DJ. Urban coccidioidomycosis and histoplasmosis: Sacramento and Indianapolis [editorial]. N Engl J Med. 1979;301:381–2. DOIPubMedGoogle Scholar
- Johnson RH, Caldwell JW, Welch G, Einstein HE. The great coccidioidomycosis epidemic: clinical features. In: Einstein HE, Catanzaro A, editors. Coccidioidomycosis: 5th International Conference. Stanford (CA): National Foundation for Infectious Diseases; 1994. p. 77-87.
- Einstein H, Johnson R, Caldwell J, Antonescu A, Reddy V. Coccidioidomycosis and pregnancy: The Kern County experience. In: Abstracts of the Interscience Conference on Antimicrobial Agents & Chemotherapy; Sept 17-20, 1995. Washington: American Society for Microbiology; 1995. p. 324.
- McCoy MJ, Ellenberg JF, Killam AP. Coccidioidomycosis complicating pregnancy. Am J Obstet Gynecol. 1980;137:739–40.PubMedGoogle Scholar
- Smale LE, Waechter KG. Dissemination of coccidioidomycosis in pregnancy. Am J Obstet Gynecol. 1970;107:356–61.PubMedGoogle Scholar
- Peterson CM, Johnson SL, Kelly JV, Kelly PC. Coccidioidal meningitis and pregnancy: a case report. Obstet Gynecol. 1989;73:835–6.PubMedGoogle Scholar
- Wack EE, Ampel NM, Galgiani JN, Bronnimann DA. Coccidioidomycosis during pregnancy. An analysis of ten cases among 47,120 pregnancies. Chest. 1988;94:376–9. DOIPubMedGoogle Scholar
- Peterson CM, Schuppert K, Kelly PC, Pappagianis D. Coccidioidomycosis and pregnancy. Obstet Gynecol Surv. 1993;48:149–56. DOIPubMedGoogle Scholar
- VanBergen WS, Fleury FJ, Cheatle EL. Fatal maternal disseminated coccidioidomycosis in a nonendemic area. Am J Obstet Gynecol. 1976;124:661–3.PubMedGoogle Scholar
- Healthcare Cost and Utilization Project: Nationwide Inpatient Sample. Rockville (MD): Agency for Healthcare Research and Quality; 1996.
- U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Alexandria (VA): International Medical Publishing; 1996.
- Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high risk population in the United States. N Engl J Med. 1980;303:833–41. DOIPubMedGoogle Scholar
- Stevens DA, Levine HB, Deresinski SC, Ten Eyck DR, Restrepo MA. Epidemiological and clinical skin testing studies with spherulin. In: Ajello L, editor. Coccidioidomycosis: Current clinical and diagnostic status. Vol. 1. Miami: Symposia Specialists; 1977. p. 107-14.
- Lebowitz MD, Johnson WM, Kaltenborn W. Coccidioidin skin test reactivity and cross-reactivity with histoplasmin in a Tucson population. In: Ajello L, editor. Coccidioidomycosis: Current clinical and diagnostic status. Vol. 1. Miami: Symposia Specialists; 1977. p. 45-61.
- Dodge RR, Lebowitz MD, Barbee R, Burrows B. Estimates of C. immitis infection by skin test reactivity in an endemic community. Am J Public Health. 1985;75:863–5. DOIPubMedGoogle Scholar
- Levine HB, Gonzalez-Ochoa A, Ten Eyck DR. Dermal sensitivity to Coccidioides immitis. A comparison of responses elicited in man by spherulin and coccidioidin. Am Rev Respir Dis. 1973;107:379–86.PubMedGoogle Scholar
- Levine HB, Restrepon A, Eyck DR, Stevens DA. Spherulin and coccidioidin: cross-reactions in dermal sensitivity to histoplasmin and paracoccidioidin. Am J Epidemiol. 1975;101:512–6.PubMedGoogle Scholar
- Gifford M, Buss W, Douds R. Annual report of the Kern County Health Department for the fiscal year July 1, 1936 to June 30, 1937.
- Edwards PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest. 1957;31:35–60.PubMedGoogle Scholar
- Hugenhotz PG. Skin test survey at Williams Air Force Base, Arizona. In: Symposium on Coccidioidomycosis, Phoenix, AZ. Washington: U.S. Government Printing Office; 1957.
- Maddy KT. The geographic distribution of Coccidioides immitis and possible ecologic implications. Ariz Med. 1958;15:178.PubMedGoogle Scholar
- Klotz AL, Biddle M. Coccidioidin skin test survey of San Fernando Valley State college students over a five year period. In: The Second Symposium on Coccidioidomycosis. Phoenix (AZ): University of Arizona Press; 1965.
- Doto I. Coccidioidin, histoplasmin, and tuberculin sensitivity among school children in Maricopa Co., Arizona. Am J Epidemiol. 1972;95:464.PubMedGoogle Scholar
- Catanzaro A. Coccidioidin sensitivity in San Diego schools. Sabouraudia. 1979;17:85–9. DOIPubMedGoogle Scholar
- Fredrich BE. A skin test survey of valley fever in Tijuana, Mexico. Soc Sci Med. 1989;29:1217–27. DOIPubMedGoogle Scholar
- Larwood T. Coccidioidin skin testing in Kern County, California: decrease in infection rate over 58 years. Clin Infect Dis. 2000;30:612–3. DOIPubMedGoogle Scholar
- Smith CE, Beard RR. Varieties of coccidiodal infection in relation to the epidemiology and control of diseases. Am J Public Health. 1946;36:1394–402. DOIGoogle Scholar
- Bayer AS. Fungal pneumonias; pulmonary coccidioidal syndromes (Part 1). Primary and progressive primary coccidioidal pneumonias --diagnostic, therapeutic, and prognostic considerations. Chest. 1981;79:575–83. DOIPubMedGoogle Scholar
- Arsura E, Caldwell J, Johnson R, Einstein H, Welch G, Talbot R, Coccidioidomycosis epidemic of 1991: epidemiologic features. In: 5th International Conference on Coccidioidomycosis. Stanford (CA) University: National Foundation for Infectious Diseases; 1994.
- Arsura EL. The association of age and mortality in coccidioidomycosis letter. J Am Geriatr Soc. 1997;45:532–3.PubMedGoogle Scholar
- Johnson RH, Caldwell JW, Welch G, Einstein HE. The great coccidioidomycosis epidemic: clinical features. In: Coccidioidomycosis: 5th International Conference. Stanford (CA) University: National Foundation for Infectious Diseases; 1994.
- Sarosi GA, Parker JD, Doto IL, Tosh FE. Chronic pulmonary coccidioidomycosis. N Engl J Med. 1970;283:325–9. DOIPubMedGoogle Scholar
- Bayer AS. Fungal pneumonias: pulmonary coccidioidal syndromes (Part 2). Miliary, nodular, and cavitary pulmonary coccidioidomycosis; chemotherapeutic and surgical considerations. Chest. 1981;79:686–91. DOIPubMedGoogle Scholar
- Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin Infect Dis. 1993;16:349–54. DOIPubMedGoogle Scholar
- Galgiani JN. Coccidioidomycosis. In: Remington JS, Swartz MN, editors. Curr Clin Top Infect Dis 1997:188-204.
- Vincent T, Galgiani John N, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces Cooperative Studies, 1955-1958. Clin Infect Dis. 1993;16:247–54. DOIPubMedGoogle Scholar
- Bouza E, Dreyer JS, Hewitt WL, Meyer RD. Coccidioidal meningitis. An analysis of thirty-one cases and review of the literature. Medicine. 1981;60:139–72.PubMedGoogle Scholar
- Galgiani JN, Catanzaro A, Cloud GA, Higgs J, Friedman BA, Larsen RA, Fluconazole therapy for coccidioidal meningitis. The NIAID-Mycoses Study Group. Ann Intern Med. 1993;119:28–35.PubMedGoogle Scholar
- Perez JA Jr, Johnson RH, Caldwell JW, Arsura EL, Nemecheck P. Fluconazole therapy in coccidioidal meningitis maintained with intrathecal amphotericin B. Arch Intern Med. 1995;155:1665–8. DOIPubMedGoogle Scholar
- Tucker RM, Denning DW, Dupont B, Stevens DA. Itraconazole therapy for chronic coccidioidal meningitis. Ann Intern Med. 1990;112:108–12.PubMedGoogle Scholar
- Catanzaro A, Galgiani JN, Levine BE, Sharkey-Mathis PK, Fierer J, Stevens DA, Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med. 1995;98:249–56. DOIPubMedGoogle Scholar
- Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF, Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med. 2000;133:676–86.PubMedGoogle Scholar
- Graybill JR, Stevens DA, Galgiani JN, Dismukes WE, Cloud GA. Itraconazole treatment of coccidioidomycosis. NAIAD Mycoses Study Group. Am J Med. 1990;89:282–90. DOIPubMedGoogle Scholar
- Oldfield EC III, Bone WD, Martin CR, Gray GC, Olson P, Schillaci RF. Prediction of relapse after treatment of coccidioidomycosis. Clin Infect Dis. 1997;25:1205–10. DOIPubMedGoogle Scholar
- Niu MT, Rhodes P, Salive M, Liveley T, Davis DM, Black S, Comparative safety data of two recombinant hepatitis B vaccines in children: data from the Vaccine Adverse Event Reporting System (VAERS) and Vaccine Safety Datalink (VSD). J Clin Epidemiol. 1998;51:503–10. DOIPubMedGoogle Scholar
- Andre FE. Summary of safety and efficacy data on a yeast derived hepatitis B vaccine. Am J Med. 1989;87:14s–20s. DOIPubMedGoogle Scholar
- Assad S. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18:57–67. DOIPubMedGoogle Scholar
- Revised 2000 National Physician Fee Schedule. Baltimore: Health Care Financing Administration; 2000.
- Manton KG, Etallard E. Chapter 6: Program payment and utilization trends for Medicare beneficiaries with disabilities. In: Wiener JM, Clauser SB, Kenner DL, editors. Persons with disabilities. Washington: The Brookings Institution; 1995. p. 117-62.
- Healthcare Cost and Utilization Project. Nationwide inpatient sample. Rockville (MD): Agency for Healthcare Research and Quality; 1996.
- Wholesale acquisition prices for pharmaceuticals in the United States. Montvale (NJ): Medical Economics; 1999.
- Gold M, Franks P, McCoy K, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36:778–92. DOIPubMedGoogle Scholar
- Gage B, Cardinalli A, Owens D. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–36. DOIPubMedGoogle Scholar
- Lipscomb J, Weinstein MC, Torrance GW. Time Preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. p. 214-35.
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 5—October 2001
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Amber E. Barnato, University of Pittsburgh School of Medicine, Division of General Internal Medicine, 933W-MUH, 200 Lothrop Street, Pittsburgh, PA 15213, USA; fax: 412-692-4838
Top