Volume 9, Number 10—October 2003
Research
Superantigens and Streptococcal Toxic Shock Syndrome
Figure 2
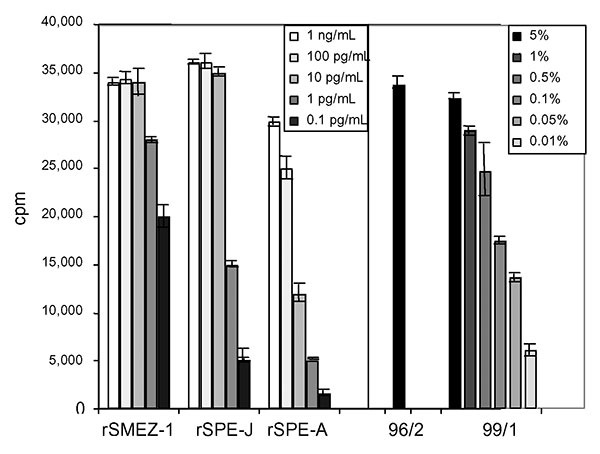
Figure 2. Mitogenic activity of acute-phase serum samples 96/2 and 99/1 compared to recombinant superantigens (SAgs). Peripheral blood lymphocytes were stimulated for 4 d with various dilutions of recombinant SAg or acute-phase serum sample 99/1. No dilution was carried out for 96/2 because of limited amount of serum. Five percent of each of the patient serum samples showed a proliferative response equal to 1–10 pg/mL of recombinant streptococcal pyrogenic exotoxin J or recombinant streptococcal mitogenic exotoxin 1. Serum 99/1 still showed significant mitogenic activity at 0.05%. SME, antistreptococcal mitogenic exotoxin; SPE, antistreptococcal pyrogenic exotoxin.
Page created: January 10, 2011
Page updated: January 10, 2011
Page reviewed: January 10, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.