Volume 9, Number 12—December 2003
Research
Intensity of Rainfall and Severity of Melioidosis, Australia
Cite This Article
Citation for Media
Abstract
In a 12-year prospective study of 318 culture-confirmed cases of melioidosis from the Top End of the Northern Territory of Australia, rainfall data for individual patient locations were correlated with patient risk factors, clinical parameters, and outcomes. Median rainfall in the 14 days before admission was highest for those dying with melioidosis (211 mm), in comparison to 110 mm for those surviving (p = 0.0002). Median 14-day rainfall was also significantly higher for those admitted with pneumonia. On univariate analysis, a prior 14-day rainfall of ≥125 mm was significantly correlated with pneumonia (odds ratio [OR] 1.70 [confidence interval [CI] 1.09 to 2.65]), bacteremia (OR 1.93 [CI 1.24 to 3.02]), septic shock (OR 1.94 [CI 1.14 to 3.29]), and death (OR 2.50 [CI 1.36 to 4.57]). On multivariate analysis, rainfall in the 14 days before admission was an independent risk factor for pneumonia (p = 0.023), bacteremic pneumonia (p = 0.001), septic shock (p = 0.005), and death (p < 0.0001). Heavy monsoonal rains and winds may cause a shift towards inhalation of Burkholderia pseudomallei.
Melioidosis, infection with Burkholderia pseudomallei, is endemic in Southeast Asia and northern Australia (1). Within the disease-endemic region, reported incidence has been increasing; melioidosis is now recognized as the most common cause of severe community-acquired sepsis in parts of northeast Thailand (2) and the most common cause of fatal community-acquired bacteremic pneumonia in the tropical “Top End” of the Northern Territory of Australia (3). The recognized endemic region for melioidosis has also been expanding, with recent reports from Taiwan (4), China, and India (1). Sporadic foci of melioidosis have occurred in temperate locations, possibly resulting from introduced infection (1,5). Melioidosis is also an important infection to consider in travelers returning from a disease- endemic region (6,7). While most cases are from recent infection with B. pseudomallei, latency is well recognized, and disease has occurred up to 29 years after a person has left a melioidosis-endemic area (8).
The association between rainfall and melioidosis has long been recognized, with 75% and 85% of cases occurring in the wet season in northeast Thailand (9) and northern Australia (3), respectively. In both regions, the number of seasonal cases correlates with total rainfall.
B. pseudomallei is an environmental bacterium of soil and surface water in disease-endemic locations. We have previously documented the incubation period for melioidosis from defined inoculating events to be 1–21 (mean 9) days (10). While most cases are considered to be from percutaneous inoculation (10,11), inhalation is also well recognized as a mode of infection. We have noted that melioidosis patients are often more severely ill after heavy monsoonal rainfall. We now show that intensity of rainfall is an independent predictor of melioidosis in persons admitted to hospital with pneumonia and of death. We postulate that heavy rainfall results in a shift towards inhalation as the mode of infection with B. pseudomallei, which leads to more severe illness.
Patients
The Darwin prospective melioidosis study has documented 318 culture-confirmed cases of melioidosis that occurred in the Top End of the Northern Territory in the 12 years from October 1989 until October 2001. Patient data are stored in Oracle software, version 8.0.4 (Oracle, North Sydney, Australia). Patient variables, as defined previously (3), include age, sex, ethnicity (aboriginal, non-aboriginal), location, and risk factors, including diabetes, alcohol excess, chronic lung disease, smoking, chronic renal disease, and kava use. Clinical parameters include nature of primary melioidosis signs and symptoms (pneumonia, other), presence of bacteremia, septic shock (presence of hypotension not responsive to fluid replacement together with hypoperfusion abnormalities manifest as end organ dysfunction) (12), and outcome (death, survival).
Rainfall Data
The Top End covers 516,945 km2. Daily rainfall data from 12 recording stations, located throughout the region and including major remote Aboriginal communities, were provided by the Bureau of Meteorology in Darwin. From these data we calculated the rainfall at each patient’s location for defined periods before date of admission. Given a mean incubation period of 9 days for acute melioidosis, we used rainfall in the 14 days before admission for each patient (14-day rainfall) to broadly reflect the rainfall exposure around the infecting event.
Statistical Analysis
Statistical analyses were performed by using Intercooled STATA 7.0 (Stata, College Station, TX). Initially, median 14-day rainfall was compared for patient variables and clinical parameters. Analysis by t tests was performed after the rainfall data were normalized by using square root transformation. Subsequently, univariate and multivariate analysis was performed with the outcomes being the various clinical parameters. Categorical variables included were age (<45 years, ≥45 years), gender, ethnicity, diabetes, alcohol excess, chronic lung disease, smoking, chronic renal disease, kava use, absence of any risk factors (those listed above or age ≥45 years or cardiac failure, malignancy, or immunosuppressive therapy) and 14-day rainfall (<125 mm, ≥125 mm). Separate multivariate analysis was also performed with normalized 14-day rainfall data as a continuous variable. All logistic regressions were performed by using stepwise forwards technique to find the most parsimonious and significant model.
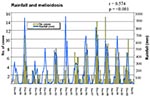
The Figure shows the close association between total monthly rainfall, as recorded at Darwin Airport, and the number of cases of melioidosis in the Top End for each month during the 12 years. The correlation between monthly cases of melioidosis and rainfall at Darwin Airport in the preceding calendar month (r = 0.617; p < 0.0001) was slightly tighter than the correlation with rainfall in the concurrent month (r = 0.574).
Table 1 shows the median 14-day rainfall correlation with various risk factors, clinical signs and symptoms, and outcomes. The correlation with median 14-day rainfall was significantly higher for patients with pneumonia, those with bacteremia and septic shock, and those who died. For those patients with pneumonia, correlation with median 14-day rainfall was significantly higher if they were bacteremic. Patients with diabetes, alcohol excess, and chronic renal disease were all associated with significantly higher median 14-day rainfall; correlation with 14-day rainfall did not significantly differ for age, sex, ethnicity, chronic lung disease, and smoking (data not shown).
Table 2 shows that, on univariate analysis, 14-day rainfall ≥125 mm correlated significantly with primary symptoms of pneumonia, bacteremia, and septic shock, and with death. Table 3 shows independent predictors of clinical signs and symptoms and outcome when multivariate analysis with 14-day rainfall as a categorical variable was used. When 14-day rainfall was used as a continuous variable, it was an independent risk factor for a admission with pneumonia (p = 0.023), bacteremic pneumonia (p = 0.001), septic shock (p = 0.005), and of death (p < 0.0001). Notably, while absence of any risk factors was a predictor of primary signs and symptoms other than pneumonia and of less severe disease, neither diabetes, alcohol excess, nor chronic renal disease was an independent predictor of signs and symptoms, disease severity, or death.
Our data confirm our observations that patients admitted with melioidosis 1–2 weeks after heavy monsoonal rainfall are more ill and more likely to die. Median rainfall in the 14 days before admission was highest in those who died with melioidosis (211 mm). For those admitted with bacteremic pneumonia, prior 14-day median rainfall was 188 mm, compared with 89 mm in patients who were nonbacteremic and did not have pneumonia. Multivariate analysis showed that rainfall in the 14 days before admission was an independent predictor of septic shock and death. Patients were 2.5 times more likely to die from melioidosis if the rainfall in the 14 days before admission was ≥125 mm. Overall, 68% of deaths occurred in this high rainfall group. Furthermore, prior heavy rainfall was an independent predictor of admission with pneumonia rather than with no pneumonia. Patients were almost twice as likely to have bacteremic pneumonia if the rainfall in the 14 days before admission was ≥125 mm.
Earlier literature, including that involving soldiers from the Vietnam War, suggests that inhalation is a common mode of infection with B. pseudomallei (13,14). This scenario was proposed for those exposed to dust raised by helicopter rotor blades in Vietnam (15). However, recent reviews have supported the predominant role of percutaneous inoculation of B. pseudomallei after exposure to muddy soils or surface water in endemic locations (10,11,16). Admissions with melioidosis pneumonia after presumptive inoculating skin injuries have been documented in patients with soil-contaminated burns (17) and are also common in our hospital (3,10). This finding suggests hematogenous spread to the lung rather than inhalation or spread from the upper respiratory tract. This finding is analogous to postprimary tuberculosis, with disease from hematogenous spread localizing in the upper lung zones, where highest alveolar oxygen tension exists (18). Moreover, septicemic melioidosis pneumonia patients are often more systemically ill than is suggested by initial chest x-ray, supporting the concept of spread to, rather than from, the lung.
Even if percutaneous inoculation is more common overall, the association of prior heavy rainfall with both pneumonia and more severe disease may well reflect a shift towards inhalation as the mode of acquiring B. pseudomallei. The periods of intense monsoonal rainfall are usually also associated with heavy winds and melioidosis cases, and outbreaks are documented after cyclonic winds and rain (19,20). Aerosolization of bacteria from surface soil and water under such conditions is probable, resulting in the potential for inhalation of B. pseudomallei. Melioidosis following near-drowning is well recognized, with aspiration considered the likely infecting event, followed by pneumonia after an incubation period as short as 2 days (21–23).
That melioidosis can potentially be more severe after inhalation than after percutaneous inoculation is not surprising. This finding is well recognized for anthrax, plague, and tularemia and has implications for biological warfare considerations (24–26). However, as with melioidosis, septicemia with pulmonary involvement after percutaneous inoculation is well recognized with anthrax, plague, and tularemia. The lack of clarity of correlation between mode of infection, site of disease, and clinical course in the melioidosis literature is also evident in descriptions of the closely related disease, glanders (infection with B. mallei) (15).
Additional possible explanations for more severe disease after heavy rainfall include a larger bacterial inoculating dose and infection with more virulent bacteria. The association of melioidosis with the wet season has also been postulated to be due to movement of B. pseudomallei from deeper soil layers to the surface with the rising water table (27). Early studies also speculated that the increased isolation of B. pseudomallei from surface water after heavy rains resulted from increased growth of the bacteria (28). More recently, the possibility has been raised that B. pseudomallei may persist in the environment in a viable nonculturable state during times of stress, such as in prolonged dry seasons (20,29). Differential gene activation likely allows such environmental bacteria to respond and adapt to different environmental conditions (30). Recently, viable but nonculturable cells of Francisella tularensis have been shown to be avirulent in mice (31). Thus, both increased environmental bacterial load and increased virulence of environmental B. pseudomallei may possibly result from periods of heavy rainfall. A possible confounder to analyzing associations of rainfall with disease severity is that, whatever the mechanisms of more severe disease, such cases will tend to have shorter incubation periods. Therefore, the prior 14-day rainfall is likely to more closely reflect the rainfall associated with infection in these cases than in less severe cases, where incubation periods >14 days might occur.
In patients with melioidosis in this study, diabetes, alcohol excess, and chronic renal disease were associated with higher prior rainfall. We previously suggested that the predisposition to melioidosis in persons with these three conditions may relate primarily to impaired polymorphonuclear leukocyte (PMNL) functions (3). This hypothesis is supported by data from an observational, uncontrolled study showing improved survival with use of granulocyte colony-stimulating factor (G-CSF) in melioidosis septic shock (32). Recent animal data suggest an important role for lung-derived G-CSF in controlling intrapulmonary infection (33). Therefore, in diabetes, alcoholism, or chronic renal disease, both impaired phagocytic activity of alveolar macrophages and impaired recruitment of PMNL into the lungs as a result of acquired dysfunction of alveolar macrophages may be critical, in addition to impaired PMNL function, in determining the predisposition to melioidosis pneumonia. Such a predisposition is likely to be especially important in influencing whether infection becomes established after inhalation of B. pseudomallei. The association of diabetes, alcohol excess, and chronic renal disease with higher prior rainfall may therefore reflect a particular susceptibility to inhalation as a mode of infection in patients with these risk factors. Alternatively, this finding may reflect a greater influence of bacterial load or organism virulence in these risk groups. Either explanation is consistent with the observation from Thailand that risk factors and level of environmental exposure to B. pseudomallei have a compound interaction, as is evident in the especially high rates of melioidosis in diabetic rice farmers (34).
We have shown that the intensity of rainfall in the 14 days before a person is admitted to hospital with melioidosis is an independent predictor of the patient’s having pneumonia, septic shock developing, and death. We postulate that this may reflect a shift towards inhalation of B. pseudomallei as the mode of transmission after heavy monsoonal rains and winds.
Dr. Currie is an infectious diseases physician at Royal Darwin Hospital, head of the Infectious Diseases Program at the Menzies School of Health Research in Darwin, and head of the Biomedical Program of the National Health and Medical Research Council–funded Cooperative Research Centre for Aboriginal and Tropical Health. His clinical and research interests focus on tropical diseases in northern Australia and the region, including melioidosis, rheumatic fever and streptococcal infections, scabies, and snakebite and jellyfish toxinology.
Acknowledgments
We acknowledge the support of all our clinical and laboratory colleagues involved in managing the melioidosis cases and thank Peter Bate from the Bureau of Meteorology in Darwin for providing rainfall data.
This study was supported by the Australian National Health and Medical Research Council, the Cooperative Research Centre for Aboriginal and Tropical Health, and the Northern Territory Clinical School.
References
- Dance DA. Melioidosis as an emerging global problem. Acta Trop. 2000;74:115–9. DOIPubMedGoogle Scholar
- Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–9.PubMedGoogle Scholar
- Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, Selva-Nayagam S, Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–6. DOIPubMedGoogle Scholar
- Hsueh PR, Teng LJ, Lee LN, Yu CJ, Yang PC, Ho SW, Melioidosis: an emerging infection in Taiwan? Emerg Infect Dis. 2001;7:428–33.PubMedGoogle Scholar
- Munckhof WJ, Mayo MJ, Scott I, Currie BJ. Fatal human melioidosis acquired in a subtropical Australian city. Am J Trop Med Hyg. 2001;65:325–8.PubMedGoogle Scholar
- Dance DA, Smith MD, Aucken HM, Pitt TL. Imported melioidosis in England and Wales. Lancet. 1999;353:208. DOIPubMedGoogle Scholar
- Visca P, Cazzola G, Petrucca A, Braggion C. Travel-associated Burkholderia pseudomallei infection (melioidosis) in a patient with cystic fibrosis: a case report. Clin Infect Dis. 2001;32:E15–6. DOIPubMedGoogle Scholar
- Chodimella U, Hoppes WL, Whalen S, Ognibene AJ, Rutecki GW. Septicemia and suppuration in a Vietnam veteran. Hosp Pract. 1997;32:219–21.PubMedGoogle Scholar
- Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–90. DOIPubMedGoogle Scholar
- Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S, Snelling PL, The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000;74:121–7. DOIPubMedGoogle Scholar
- Leelarasamee A, Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989;11:413–25.PubMedGoogle Scholar
- American College of Chest Physicians/Society of Critical Care Medicine. Consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. DOIPubMedGoogle Scholar
- Brundage WG, Thuss CJJ, Walden DC. Four fatal cases of melioidosis in U. S. soldiers in Vietnam. Bacteriologic and pathologic characteristics. Am J Trop Med Hyg. 1968;17:183–91.PubMedGoogle Scholar
- Mackowiak PA, Smith JW. Septicemic melioidosis. Occurrence following acute influenza A six years after exposure in Vietnam. JAMA. 1978;240:764–6. DOIPubMedGoogle Scholar
- Howe C, Sampath A, Spotnitz M. The pseudomallei group: a review. J Infect Dis. 1971;124:598–606.PubMedGoogle Scholar
- Dance DAB. Melioidosis. Reviews in Medical Microbiology. 1990;1:143–50.
- Flemma RJ, DiVincenti FC, Dotin LN, Pruitt BAJ. Pulmonary melioidosis; a diagnostic dilemma and increasing threat. Ann Thorac Surg. 1969;7:491–9. DOIPubMedGoogle Scholar
- Citron KM, Girling DJ. Tuberculosis. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. Oxford textbook of medicine. Oxford: Oxford University Press; 1987. p. 5.285–6.
- Maegraith BG, Leithead CS. Melioidosis: a case report. Lancet. 1964;1:862–3. DOIPubMedGoogle Scholar
- Inglis TJ, Mee B, Chang B. The environmental microbiology of melioidosis. Rev Med Microbiol. 2001;12:13–20.
- Achana V, Silpapojakul K, Thininta W, Kalnaowakul S. Acute Pseudomonas pseudomallei pneumonia and septicemia following aspiration of contaminated water: a case report. Southeast Asian J Trop Med Public Health. 1985;16:500–4.PubMedGoogle Scholar
- Lee N, Wu JL, Lee CH, Tsai WC. Pseudomonas pseudomallei infection from drowning: the first reported case in Taiwan. J Clin Microbiol. 1985;22:352–4.PubMedGoogle Scholar
- Pruekprasert P, Jitsurong S. Case report: septicemic melioidosis following near drowning. Southeast Asian J Trop Med Public Health. 1991;22:276–8.PubMedGoogle Scholar
- Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–52. DOIPubMedGoogle Scholar
- Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–90. DOIPubMedGoogle Scholar
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–73. DOIPubMedGoogle Scholar
- Thomas AD, Forbes Faulkner J, Parker M. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am J Epidemiol. 1979;110:515–21.PubMedGoogle Scholar
- Strauss JM, Groves MG, Mariappan M, Ellison DW. Melioidosis in Malaysia. II. Distribution of Pseudomonas pseudomallei in soil and surface water. Am J Trop Med Hyg. 1969;18:698–702.PubMedGoogle Scholar
- Brook MD, Currie B, Desmarchelier PM. Isolation and identification of Burkholderia pseudomallei from soil using selective culture techniques and the polymerase chain reaction. J Appl Microbiol. 1997;82:589–96.PubMedGoogle Scholar
- Woods DE. The use of animal infection models to study the pathogenesis of melioidosis and glanders. Trends Microbiol. 2002;10:483–4. DOIPubMedGoogle Scholar
- Forsman M, Henningson EW, Larsson E, Johansson T, Sandstrom G. Francisella tularensis does not manifest virulence in viable but non-culturable state. FEMS Microbiol Ecol. 2000;31:217–24. DOIPubMedGoogle Scholar
- Stephens DP, Fisher DA, Currie BJ. An audit of the use of granulocyte colony-stimulating factor in septic shock. Intern Med J. 2002;32:143–8. DOIPubMedGoogle Scholar
- Quinton LJ, Nelson S, Boe DM, Zhang P, Zhong Q, Kolls JK, The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. J Infect Dis. 2002;185:1476–82. DOIPubMedGoogle Scholar
- Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–13. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 9, Number 12—December 2003
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Bart Currie, Menzies School of Health Research, P.O. Box 41096 Casuarina, Northern Territory 0811, Australia; fax: 61-8-89275187
Top