Volume 13, Number 9—September 2007
Dispatch
Coronavirus Antibodies in African Bat Species
Figure 2
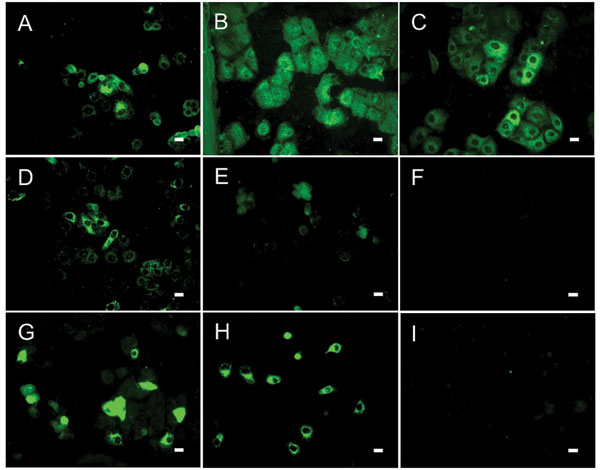
Figure 2. Results of indirect immunofluorescence (IF) test with Vero E6 cells infected with severe acute respiratory syndrome–associated coronavirus (SARS-CoV). The SARS-CoV diagnostic IIFT kit (EUROIMMUN AG, Lübeck, Germany) was used with minor modifications: bat and reference human serum specimens were diluted 1:100 (found to be the optimal dilution for bat sera) in sample buffer, and secondary detection was performed with goat-antibat immunoglobulin (Ig) (Bethyl, Montgomery, AL, USA) followed by fluorescein isothiocyanate (FITC)–labeled donkey-antigoat Ig (Dianova, Hamburg, Germany) (A–F) or FITC-labeled goat-antihuman Ig (G–I). Frames A–D, SARS-CoV ELISA–positive bat serum specimens 2, 17, 26, 31; E–F, ELISA-negative bat serum specimens 38 (showing unspecific signals) and 306; G–H, SARS-CoV–positive human control serum specimens A and B; I, negative human serum C. All photographs were taken at equivalent microscope settings. Scale bars represent 20 μm.