Volume 15, Number 2—February 2009
Research
Oseltamivir-Resistant Influenza Viruses A (H1N1), Norway, 2007–08
Figure 1
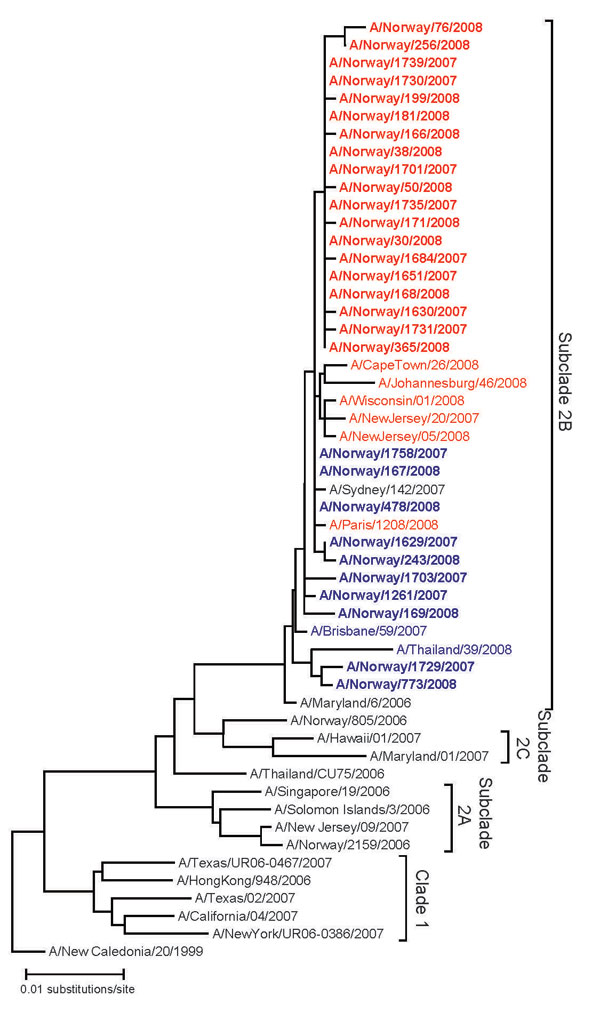
Figure 1. Phylogenetic reconstruction of the H1 genes of influenza viruses A (H1N1) in Norway, 2007–08 season. The analysis was performed on an alignment spanning positions 84–1054 of viral RNA segment 4. Pairwise distances were calculated by using the Kimura 2-parameter model with a transition:transversion ratio of 2.0; the phylogenetic tree was constructed by the neighbor-joining method, as implemented in the programs DNADIST and NEIGHBOR in the PHYLIP package (14 15,). Published sequences were obtained from the Influenza Sequence Database, Los Alamos National Laboratory (16). Boldface indicates viruses from the 2007–08 influenza season in Norway; red indicates oseltamivir-resistant viruses; blue, susceptible viruses. New sequences presented in this analysis have been deposited in GenBank (accession nos. CY036664–CY036694).
References
- McKimm-Breschkin J, Trivedi T, Hampson A, Hay A, Klimov A, Tashiro M, Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob Agents Chemother. 2003;47:2264–72. DOIPubMedGoogle Scholar
- Aavitsland P, Hauge S, Borgen K. Rare usage of oseltamivir in Norway prior to emergence of oseltamivir resistant influenza A(H1N1) virus in the 2007–2008 season. 2008 International Conference on Emerging Infectious Disases; 2008 Mar 16–19; Atlanta. Addendum 11.
- Escuret V, Frobert E, Bouscambert-Duchamp M, Sabatier M, Grog I, Valette M, Detection of human influenza A (H1N1) and B strains with reduced sensitivity to neuraminidase inhibitors. J Clin Virol. 2008;41:25–8. DOIPubMedGoogle Scholar
- Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006;50:2395–402. DOIPubMedGoogle Scholar
- Zambon M, Hayden FG. Position statement: global neuraminidase inhibitor susceptibility network. Antiviral Res. 2001;49:147–56. DOIPubMedGoogle Scholar
- World Health Organization. International Health Regulations (IHR) 2005, 2nd ed. [cited 2008 December 8]. Available from http://www.who.int/csr/ihr/IHR_2005_en.pdf
- Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay A, Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 2008;13. pii: 8026.
- Mishin VP, Hayden FG, Gubareva LV. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob Agents Chemother. 2005;49:4515–20. DOIPubMedGoogle Scholar
- Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leaves virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55:307–17. DOIPubMedGoogle Scholar
- Lackenby A, Democratis J, Siqueira M, Zambon M. Rapid quantitation of neuraminidase inhibitor drug resistance in influenza virus quasispecies. Antivir Ther. 2008;809–20.PubMedGoogle Scholar
- Wetherall NT, Trivedi T, Zeller J, Hodges-Savola C, McKimm-Breschkin JL, Zambon M, Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J Clin Microbiol. 2003;41:742–50. DOIPubMedGoogle Scholar
- Fouchier RA, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, Osterhaus AD. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–101.PubMedGoogle Scholar
- Norwegian Institute of Public Health. The 2007/2008 influenza season in Norway [cited 2008 May 28]. Available from http://www.fhi.no/eway/default.aspx?pid=238&trg=MainLeft_5895&MainArea_5811=5895:0:15,2820:1:0:0:0:0&MainLeft_5895=5825:66508:1:5896:3:0:0
- Felsenstein J. PHYLIP (phylogeny inference package) version 3.2. Cladistics. 1989;5:164–6.
- Felsenstein J. PHYLIP (phylogeny inference package) version 3.5c. Seattle: Department of Genetics, University of Washington; 1993.
- Macken C, Lu H, Goodman J, Boykin L. The value of a database in surveillance and vaccine selection. In: Osterhaus ADME, Cox N, Hampson AW, editors. Options for the control of influenza IV. Amsterdam: Elsevier Science; 2001. p. 103–6.
- European Centre for Disease Prevention and Control. Antivirals and antiviral resistance influenza [cited 2008 May 28]. Available from http://ecdc.europa.eu/en/Health_topics/influenza/antivirals_table.aspx
- World Health Organization. Influenza A(H1N1) virus resistance to oseltamivir—last quarter 2007 to 5 May 2008 [cited 2008 May 5]. Available from http://www.who.int/csr/disease/influenza/H1N1ResistanceWeb20080505.pdf
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2009 southern hemisphere influenza season [cited 2008 October 5]. Available from http://www.who.int/entity/csr/disease/influenza/200809Recommendation.pdf
- World Health Organization. Seasonal influenza activity in the world, 2008 [cited 2008 July 24]. Available from http://www.who.int/csr/disease/influenza/update/en/index.html
- World Health Organization. Influenza A(H1N1) virus resistance to oseltamivir [cited 2008 July 18]. Available from http://www.who.int/csr/disease/influenza/h1n1_table/en/index.html
- Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320:340–6. DOIPubMedGoogle Scholar
- Herlocher ML, Carr J, Ives J, Elias S, Truscon R, Roberts N, Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antiviral Res. 2002;54:99–111. DOIPubMedGoogle Scholar
- Abed Y, Baz M, Boivin G. Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir Ther. 2006;11:971–6.PubMedGoogle Scholar
- Yen HL, Ilyushina NA, Salomon R, Hoffmann E, Webster RG, Govorkova EA. Neuraminidase inhibitor-resistant recombinant A/Vietnam/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J Virol. 2007;81:12418–26. DOIPubMedGoogle Scholar
- Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog. 2008;4:e1000103. DOIPubMedGoogle Scholar