Volume 4, Number 1—March 1998
Synopsis
Diversity among Multidrug-Resistant Enterococci
Figure 2
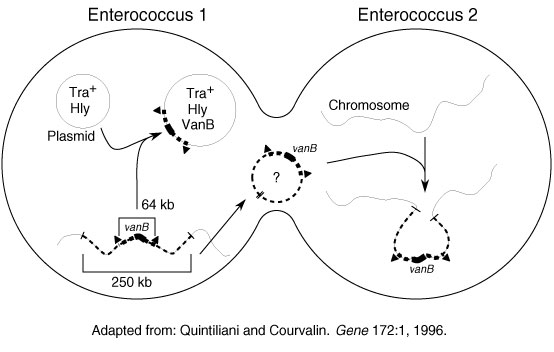
Figure 2. Potential modes of spread of vancomycin-resistant genes. Adapted in part from Quintiliani and Courvalin (14).
References
- Murray BE. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65.PubMedGoogle Scholar
- Moellering RC Jr. Emergence of enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–8.PubMedGoogle Scholar
- Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(Suppl 3B):72S–5S. DOIPubMedGoogle Scholar
- Hodges TL, Zighelboim-Daum S, Eliopoulos GM, Wennersten C, Moellering RC Jr. Antimicrobial susceptibility changes in Enterococcus faecalis following various penicillin exposure regimens. Antimicrob Agents Chemother. 1992;36:121–5.PubMedGoogle Scholar
- Zervos MJ, Schaberg DS. Reversal of the in vitro susceptibility of enterococci to trimethoprim-sulfamethoxazole by folinic acid. Antimicrob Agents Chemother. 1985;28:446–8.PubMedGoogle Scholar
- Najjar A, Murray BE. Failure to demonstrate a consistent in vitro bactericidal effect of trimethoprim-sulfamethoxazole against enterococci. Antimicrob Agents Chemother. 1987;31:808–10.PubMedGoogle Scholar
- Chenoweth CE, Robinson KA, Schaberg DR. Efficacy of ampicillin versus trimethprim-sulfamethoxazole in a mouse model of lethal enterococcal peritonitis. Antimicrob Agents Chemother. 1990;34:1800–2.PubMedGoogle Scholar
- Grayson ML, Thauvin-Eliopoulos C, Eliopoulos GM, Yao JDC, DeAngelis DV, Walton L, Failure of trimethoprim-sulfamethoxazole therapy in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1990;34:1792–4.PubMedGoogle Scholar
- Clewell DB, Keith EW. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989;21:175–84. DOIPubMedGoogle Scholar
- Clewell DB. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981;45:409–36.PubMedGoogle Scholar
- Clewell DB. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–59. DOIPubMedGoogle Scholar
- Roberts MC. Characterization of the Tet M determinants in urogenital and respiratory bacteria. Antimicrob Agents Chemother. 1990;34:476–8.PubMedGoogle Scholar
- Roberts MC, Hillier SL. Genetic basis of tetracycline resistance in urogenital bacteria. Antimicrob Agents Chemother 1990;34:261-4.13
- Quintiliani R Jr, Courvalin P. Characterization of Tn1547, a composite transposon flanked by the IS16 and IS256-like elements, that confers vancomycin resistance in Enterococcus faecalis BM4281. Gene 1996;172:1-8.14
- Ferretti JJ, Gilmore KS, Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986;167:631–8.PubMedGoogle Scholar
- Carlier C, Courvalin P. Emergence of 4',4"-aminoglycoside nucleotidyltransferase in enterococci. Antimicrob Agents Chemother. 1990;34:1565–9.PubMedGoogle Scholar
- Chow JW, Zervos MJ, Lerner SA, Thal LA, Donabedian SM, Jaworski DD, A novel gentamicin resistance gene in Enterococcus. Antimicrob Agents Chemother. 1997;41:511–4.PubMedGoogle Scholar
- Murray BE. ß-lactamase-producing enterococci. Antimicrob Agents Chemother. 1992;36:2355–9.PubMedGoogle Scholar
- Murray BE, Singh KV, Markowitz SM, Lopardo HA, Patterson JE, Zervos MJ, Evidence for clonal spread of a single strain of ß-lactamase-producing Enterococcus faecalis to six hospitals in five states. J Infect Dis. 1991;163:780–5.PubMedGoogle Scholar
- Rhinehart E, Smith NE, Wennersten C, Gorss E, Freeman J, Eliopoulos GM, Rapid dissemination of ß-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990;323:1814–8.PubMedGoogle Scholar
- Wells VD, Wong ES, Murray BE, Coudron PE, Williams DS, Markowitz DM. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992;116:285–92.PubMedGoogle Scholar
- Grayson ML, Eliopoulos GM, Wennersten CB, Ruoff KL, de Girolami PC, Ferraro M-J, Increasing resistance to ß-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob Agents Chemother. 1991;35:2180–4.PubMedGoogle Scholar
- Fontana R, Ligozzi M, Pittaluga F, Satta G. Intrinsic penicillin resistance in enterococci. Microb Drug Resist. 1996;2:209–13. DOIPubMedGoogle Scholar
- Rice LB, Carias LL, Rudin SB, Donskey CJ. Abstract #LB-17 Transferable ampicillin resistance in Enterococcus faecium and linkage of ampicillin and vancomycin-resistance determinants [abstract]. In program of the 37th ICAAC Program Addendum; 1997 Sep 28—Oct1; Toronto, Ontario, Canada.
- Perichon B, Reynolds P, Van Courvalin P. D-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob Agents Chemother. 1997;41:2016–8.PubMedGoogle Scholar
- Derlot E, Courvalin P. Mechanisms and implications of glycopeptide resistance in enterococci. Am J Med. 1991;91(Suppl 3B):82S–5S. DOIPubMedGoogle Scholar
- Arthur M, Molinas C, Bugg TDH, Wright GD, Walsh CT, Courvalin P. Evidence for in vivo incorporation of D-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992;36:867–9.PubMedGoogle Scholar
- Dutka-Malen S, Molinas C, Arthur M, Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992;112:53–8. DOIPubMedGoogle Scholar
- Navarro F, Courvalin P. Analysis of genes encoding D-alanine-D-alanine ligase-related enzymes in Enterococcus casseliflavus and Enterococcus flavescens. Antimicrob Agents Chemother. 1994;38:1788–93.PubMedGoogle Scholar
- Billot-Klein D, Gutmann L, Sable S, Guittet E, van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994;176:2398–405.PubMedGoogle Scholar
- Moellering RC Jr, Wennersten C, Medrek T, Weinberg AN. Prevalence of high-level resistance to aminoglycosides in clinical isolates of enterococci. Antimicrobial agents and chemotherapy--1970. Washington. American Society for Microbiology. 1971;1971:335–40.
- Watanakunakorn C. The prevalence of high-level aminoglycoside resistance among enterococci isolated from blood cultures during 1980-1988. J Antimicrob Chemother. 1989;24:63–8. DOIPubMedGoogle Scholar
- Phillips I, King A, Gransden WR, Eykyn SJ. The antibiotic sensitivity of bacteria isolated from the blood of patients in St. Thomas' Hospital, 1969-1988. J Antimicrob Chemother. 1990;25:59–80.PubMedGoogle Scholar
- Zervos MJ, Dembinski S, Mikesell T, Schaberg DR. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986;153:1075–83.PubMedGoogle Scholar
- Eliopoulos GM, Wennersten C, Zighelboim-Daum S, Reiszner E, Goldmann D, Moellering RC Jr. High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988;32:1528–32.PubMedGoogle Scholar
- Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–63.PubMedGoogle Scholar
- Zervos MJ, Mikesell TS, Schaberg DR. Heterogeneity of plasmids determining high-level resistance to gentamicin in clinical isolates of Streptococcus faecalis. Antimicrob Agents Chemother. 1986;30:78–81.PubMedGoogle Scholar
- Hodel-Christian SL, Murray BE. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991;35:1147–52.PubMedGoogle Scholar
- Hodel-Christian SL, Smith M, Zscheck KZ, Murray BE. 1991. Comparison of a gentamicin resistance transposon and a beta-lactamase gene from enterococci to those from staphylococci. In: Dunny GM, Cleary PP, McKay LL, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington: American Society for Microbiology; 1991. p. 54-8.
- Thal LA, Chow JW, Clewell DB, Zervos MJ. Tn924, a chromosome-borne transposon encoding high-level gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1994;38:1152–6.PubMedGoogle Scholar
- Rice LB, Carias LL, Marshall SH, Bonafede ME. Sequences found on staphylococcal ß-lactamase plasmids integrated into the chromosome of Enterococcus faecalis CH116. Plasmid. 1996;35:81–90. DOIPubMedGoogle Scholar
- Seetulsingh PS, Tomayko JF, Coudron PE, Markowitz SM, Skinner C, Singh KV, Chromosomal DNA restriction endonuclease digestion patterns of ß-lactamase-producing Enterococcus faecalis isolates collected from a single hospital over a 7-year period. J Clin Microbiol. 1996;34:1892–6.PubMedGoogle Scholar
- Tomayko JF, Murray BE. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J Clin Microbiol. 1995;33:2903–7.PubMedGoogle Scholar
- Coudron PE, Markowitz SM, Wong ES. Isolation of a ß-lactamase-producing, aminoglycoside-resistant strain of Enterococcus faecium. Antimicrob Agents Chemother 1992;36:1125-6.44
- Patterson JE, Singh KV, Murray BE. Epidemiology of an endemic strain of ß-lactamase-producing Enterococcus faecalis. J Clin Microbiol 1991;29:2513-6.45
- Handwerger S, Perlman DC, Altarac D, McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis 1992;14:655-61.46
- Miranda AG, Singh KV, Murray BE. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol 1991;29:2752-7.47
- Handwerger S, Skoble J. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1995;39:2446–53.PubMedGoogle Scholar
- Handwerger S, Skoble J, Discotto LF, Pucci MJ. Heterogeneity of the vanA gene cluster in clinical isolates of enterococci from the Northeastern United States. Antimicrob Agents Chemother. 1995;39:362–8.PubMedGoogle Scholar
- van den Bogaard AE, Jensen LB, Stobberingh EE. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med. 1997;337:1558–9. DOIPubMedGoogle Scholar
- Power EGM, Abdulla YH, Talsania HG, Spice W, Aathithan S, French GL. vanA genes in vancomycin-resistant clinical isolates of Oerskovia turbata and Arcanobacterium (Corynebacterium) haemolyticum. J Antimicrob Chemother. 1995;36:595–606. DOIPubMedGoogle Scholar
- Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob Agents Chemother. 1997;41:24–9.PubMedGoogle Scholar
- Biavasco F, Miele A, Vignaroli C, Manso E, Lupid R, Varaldo PE. Genotypic characterization of a nosocomial outbreak of VanA Enterococcus faecalis. Microb Drug Resist. 1996;2:231–7. DOIPubMedGoogle Scholar
- Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter-Bynoe G, Sherman CB, Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–53.PubMedGoogle Scholar
- Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh KV, Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin and gentamicin. Clin Infect Dis. 1993;16:750–5.PubMedGoogle Scholar
- Livornese LL Jr, Dias S, Samel C, Romanowski B, Taylor S, May P, Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–6.PubMedGoogle Scholar
- Moreno F, Grota P, Crisp C, Magnon K, Melcher GP, Jorgensen JH, Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin Infect Dis. 1995;21:1234–7.PubMedGoogle Scholar
- Perlada DE, Smulian AG, Cushion MT. Molecular epidemiology and antibiotic susceptibility of enterococci in Cincinnati, Ohio: a prospective citywide survey. J Clin Microbiol. 1997;35:2342–7.PubMedGoogle Scholar
- Chow JW, Kuritza A, Shlaes DM, Green M, Sahm DF, Zervos MJ. Clonal spread of vancomycin-resistant Enterococcus faecium between patients in three hosptials in two states. J Clin Microbiol. 1993;31:1609–11.PubMedGoogle Scholar
- Clark NC, Cooksey RC, Hill BC, Swenson JM, Tenover FC. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–7.PubMedGoogle Scholar
- Sader HS, Pfaller MA, Tenover FC, Hollis RJ, Jones RN. Evaluation and characterization of multiresistant Enterococcus faecium from 12 U.S. medical centers. J Clin Microbiol 1994;32:2840-2.61
- Boyle JF, Soumakis SA, Rendo A, Herrington JA, Gianarkis DG, Thurberg BE, Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol. 1993;31:1280–5.PubMedGoogle Scholar
- Morris JG Jr, Shay DK, Hebden JN, McCarter RJ Jr, Perdue BE, Jarvis W, Enterococci resistant to multiple antimicrobial agents, including vancomycin. Ann Intern Med. 1995;123:250–9.PubMedGoogle Scholar
- Bingen EH, Denamur E, Lambert-Zechovsky NY, Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991;29:1888–92.PubMedGoogle Scholar
- Plessis P, Lamy T, Donnio PY, Autuly F, Grulois I, LePrise PY, Epidemiologic analysis of glycopeptide-resistant Enterococcus strains in neutropenic patients receiving prolonged vancomycin administration. Eur J Clin Microbiol Infect Dis. 1995;14:959–63. DOIPubMedGoogle Scholar
- Jordens JZ, Bates J, Griffiths DT. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1994;34:515–28. DOIPubMedGoogle Scholar
- Klare I, Heier H, Claus H, Bohme G, Marin S, Seltmann G, Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb Drug Resist. 1995;3:265–72. DOIGoogle Scholar
- Van der Auwera P, Pensart N, Korten V, Murray BE, Leclercq R. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly-glycopeptide-resistant enterococci. J Infect Dis. 1995;173:1129–36.
- Aarestrup FM, Ahrens P, Madsen M, Pallesen LV, Poulsen RL, Westh H. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob Agents Chemother. 1996;40:1938–40.PubMedGoogle Scholar
- Bates J, Jordens Z, Selkon JB. Evidence for an animal origin of vancomycin-resistant enterococci [letter]. Lancet. 1993;342:490. DOIPubMedGoogle Scholar
- Klare I, Heier H, Claus H, Reissbrodt R, Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–72. DOIPubMedGoogle Scholar
- Schouten MA, Voss A, Hoogkamp-Korstanje JAA. VRE and meat. Lancet. 1997;349:1258. DOIPubMedGoogle Scholar
- Coque TM, Tomayko JF, Ricke SC, Okhuysen PO, Murray BE. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–9.PubMedGoogle Scholar
- Kjerulf A, Pallesen L, Westh H. Vancomycin-resistant enterococci at a large university hospital in Denmark. APMIS. 1996;104:475–9. DOIPubMedGoogle Scholar
- Klare I, Heier H, Claus H, Witte W. Environmental strains of Enterococcus faecium with inducible high-level resistance to glycopeptides. FEMS Microbiol Lett. 1993;106:23–30. DOIPubMedGoogle Scholar
The reference 52 "Poyart, Pierre, Quesne, Pron, Berche, Trieu-Cuot, 1997" is not cited in the text. Please add an in-text citation or delete the reference.
The reference 66 "Jordens, Bates, Griffiths, 1994" is not cited in the text. Please add an in-text citation or delete the reference.
Page created: December 13, 2010
Page updated: December 13, 2010
Page reviewed: December 13, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.