Volume 5, Number 2—April 1999
Synopsis
Enteropathogenic E. coli, Salmonella, and Shigella: Masters of Host Cell Cytoskeletal Exploitation
Figure 3
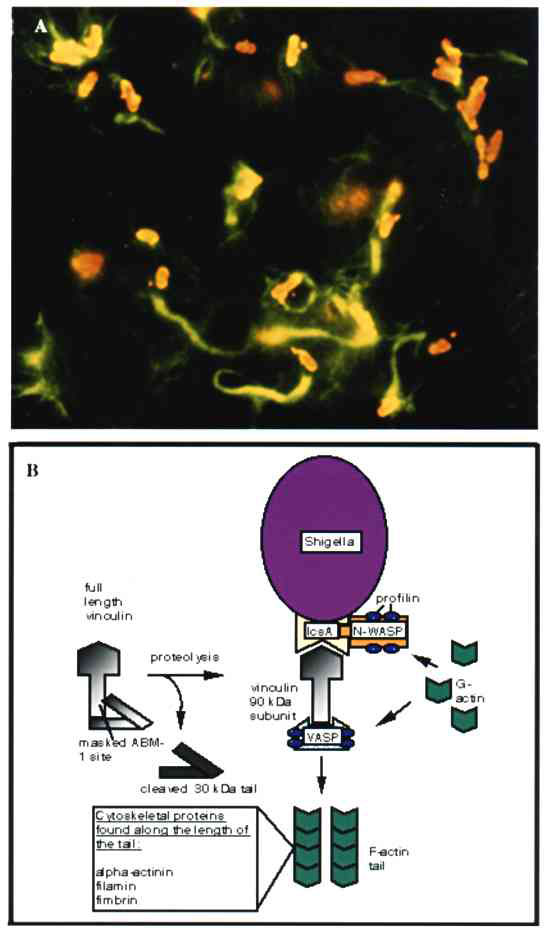
Figure 3. A. Immunofluorescence micrograph showing Shigella (red) propelling itself through the cytoplasm by polymerizing actin (green) (Philippe Sansonetti, Institut Pasteur, reprinted with permission from Trends in Microbiology, 1996). B. Shigella-mediated cytoskeletal rearrangements. The outer membrane protein, IcsA, is sufficient to drive actin-based motility of Shigella. IcsA directly binds two proteins, vinculin and neural-Wiskott-Aldrich Syndrome protein (N-WASP). Vinculin undergoes proteolysis within the host cell upon Shigella infection, producing a 90-kDa fragment that can bind to IcsA and to the vasodilator-stimulated phosphoprotein (VASP). VASP in turn can recruit profilin to the bacterial surface, which can provide actin for tail construction. N-WASP binding of IcsA can also recruit profilin to the bacterial surface and may be another means of obtaining monomeric actin for tail formation and subsequent bacterial motility.