Volume 11, Number 12—December 2005
Perspective
Community Epidemiology Framework for Classifying Disease Threats
Cite This Article
Citation for Media
Abstract
Recent evidence suggests that most parasites can infect multiple host species and that these are primarily responsible for emerging infectious disease outbreaks in humans and wildlife. However, the ecologic and evolutionary factors that constrain or facilitate such emergences are poorly understood. We propose a conceptual framework based on the pathogen's between- and within-species transmission rates to describe possible configurations of a multihost-pathogen community that may lead to disease emergence. We establish 3 dynamic thresholds separating 4 classes of disease outcomes, spillover, apparent multihost, true multihost, and potential emerging infectious disease; describe possible disease emergence scenarios; outline the population dynamics of each case; and clarify existing terminology. We highlight the utility of this framework with examples of disease threats in human and wildlife populations, showing how it allows us to understand which ecologic factors affect disease emergence and predict the impact of host shifts in a range of disease systems.
Models of host-pathogen dynamics have typically assumed a single-host population infected by a single pathogen. However, most pathogens can infect several host species; >60% of human pathogens, >68% of wild primate parasites, and >90% of domesticated animal pathogens infect multiple host species (1–3). An interest in multihost pathogens is particularly timely, given that many of the most threatening current pathogens (e.g., HIV, West Nile virus, influenza virus, Ebola virus) are believed to have crossed species barriers to infect humans, domesticated animals, or wildlife populations (1,3–8). However, we do not know the host and pathogen characteristics that determine such host shifts and the likely characteristics of future emerging infectious diseases. To address this issue, 2 theoretical approaches have been adopted. The first, using dynamic models, focuses on the host's perspective and ascertains how a shared pathogen affects the dynamics of 2 host populations (9–12). The second approach takes the pathogen's point of view and considers how combined host densities affect pathogen persistence within the community (13–15). However, as the number of studies grows, so does the terminology. Terms such as multihost pathogens, dead-end hosts, reservoir hosts, host shifts, and spillovers are frequently used, but often different phrases are used to describe the same phenomenon, and possibly more concerning, the same terminology may be used to describe strictly different phenomena.
This lack of consolidation makes it unclear how these different approaches relate in terms of understanding the mechanisms driving disease emergence. A need exists for a single, comprehensive framework that characterizes disease outcomes based on biologically meaningful processes. Recently, attempts have been made to reconcile these concepts, mainly by highlighting the role of reservoir hosts (13,16). Haydon et al. (13) proposed a conceptual model that assumed a target host species was exposed to a pathogen endemic in a second host species (or species complex). The outcome of infection then depended on the sizes of the populations and whether they were able to maintain the pathogen alone. This approach expanded the naive view that reservoirs are nonpathogenic, single-species populations and encompassed the complexity of pathogen-host communities observed in nature. However, focusing just on host density ignores many key features of emerging diseases. The likelihood of disease emergence will depend on highly dynamic processes determined by both between- and within-species transmission rates. Therefore, ecologic forces acting on both hosts and pathogens will influence the contact structure of the community and affect the likelihood and persistence of an emerging infectious disease in a new host.
We propose a conceptual framework to describe the configurations of a host-pathogen community that may lead to disease emergence in a target host. We develop our framework from a simple 2-host 1-pathogen model and establish thresholds for pathogen and host persistence based on the between- and within-species net transmission rates. We then consider what ecologic factors determine the location of various host-pathogen systems within the framework. Finally, we use a stochastic model to consider what characteristics of the hosts and pathogen define the dynamics and likelihood of an emerging infectious disease.
We start by considering the assembly of a 2-host community infected by a single pathogen (15,17,18) where the pathogen is endemic within host population H1 such that individuals of H1 are either susceptible (S1) or infected (I1). We then assume a second target host population (H2) enters the community and can become infected by the pathogen (Figure 1A). Since the pathogen is well established in H1, we assume S1 and I1 are unchanged by H2; thus, our model most closely resembles the asymmetric model of Dobson (15). In the terminology of Haydon et al. (12), H1 is a maintenance host species (or species complex) with the potential to be a disease reservoir for H2. H2 may or may not be a maintenance host (see below). The model is
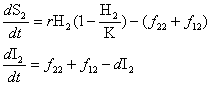 (model 1)
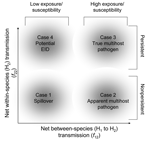
(model 1)The target host population H2 has 4 possible outcomes: 1) uninfected, 2) infected but unable to sustain the pathogen, 3) infected and able to sustain the pathogen, or 4) infected and driven to extinction by the pathogen (Figure 1). These 4 outcomes are separated by 3 thresholds (Figure 1C): i) invasion threshold, ii) persistence threshold, and iii) host extinction threshold. The first 2 thresholds are analogous to established density-based thresholds in epidemiology; the first allows ecologic invasion of a pathogen, which subsequently dies out, and the second allows persistence of the pathogen (19). Here we combine these density effects with the per capita rates of infection to express these thresholds in terms of the magnitude of the net between- and within-species transmission rates (f12 and f22, respectively).
Infection of H2 by H1 and transmission within H2 are 2 separate processes determined by f12 and f22. Different combinations of these parameters lead to the different outcomes described above, and all possible scenarios can be placed within a 2-dimensional continuum (Figure 2), with f12 on one axis (i.e., can H2 get infected from H1?) and f22 on the other (i.e., can H2 sustain infection?). We can then divide the f12 – f22 parameter space into regions of different disease outcomes.
Case 1: Spillover
In this case, the within-H2 transmission rate is too low to sustain the pathogen (f22 → 0). The between-species transmission from H1 is also low (f12 → 0). Thus, although infections of H2 do occasionally occur, they are transient. This represents the case in which the pathogen is specialized to the endemic host and there is either very low exposure to H2 (an ecologic constraint, such as parasite transmission mode) or H2 is resistant to infection (a physiologic constraint). We recommend the term spillover to describe this form of cross-species infection. Previously, spillover has been used to describe a wide range of dynamics (20), but we recommend limiting its use to transient infections in a target host because of transmission from a reservoir host that is not self-sustaining in the target population.
The recent outbreak of West Nile encephalitis in the United States is such a spillover: the virus moved from bird populations (H1) to infect humans (H2), which are unable to transmit the pathogen (β22= 0) (21). Nevertheless, spillovers still represent a serious health concern; increases in the reservoir population may lead to dramatic increases in disease prevalence in the target host.
Case 2: Apparent Multihost Pathogen
In this case, the within-species transmission rate for the target host is low, but the between-species transmission rate exceeds the invasion threshold, resulting in persistent infections in H2. This case represents apparent multihost dynamics that differ from spillover dynamics in that the disease is nontransient in H2, but the pathogen is sustained because of frequent between-species transmission from the disease-endemic host. Apparent multihost dynamics exist because the potentially high prevalence in the target host would give the appearance of a true multihost pathogen, but the lack of within-species transmission means the disease cannot be maintained in the absence of H1. We recommend the term reservoir to describe H1 in both cases 1 and 2, in which the pathogen is permanently maintained in H1 and without between-species transmission (β12), the disease would not persist in the target host.
An example of an apparent multihost pathogen is rabies in side-striped jackals (H2) in Africa. Until a recent analysis (22), rabies was considered sustainable in the jackal population (H2), but detailed monitoring showed that rabies is not self-sustaining because of the density of the low susceptible jackal population (S2), and epidemics are frequently seeded from the domestic dog reservoir (high β12).
Case 3: True Multihost Pathogen
In this case, both the within- and between-species transmission rates are high. Thus, since the pathogen can independently persist in either host population in the absence of the other, following Haydon et al (13), both are considered maintenance hosts. This case represents a true multihost pathogen with substantial within- and between-species transmission. One example is brucellosis infections around Yellowstone National Park, where the pathogen can be endemically maintained in cattle, bison, and elk populations (23).
Case 4: Potential Emerging Infectious Disease
In this case, the within-H2 transmission rate is high, but the between-species transmission rate is very low (f12 → 0). Thus, the pathogen can persist in the target host (H2), but the net rate of between-species transmission is so low that H2 is rarely exposed to the disease. This case might occur when a disease is transmitted through close contact and thus has little chance of transmission between species. Similarly, the barrier to infection could be an ecologic factor, such as geographic isolation, which may be overcome by an anthropogenic change such as the introduction of exotic or invasive species. Thus, this case represents a potential emerging infectious disease in which the pathogen will become self-sustaining in H2 once the initial barrier to infection has been crossed. This case may be the region of greatest future concern since a single transmission event can have devastating consequences because of the high rate of within-species transmission in the target host.
Recent examples of potential emerging infectious diseases that were realized include the emergence of HIV-1 and HIV-2 in human populations, in which the close-contact nature of the infection process prevented transmission of simian immunodeficiency virus (SIV) from primates to humans (6,24). Another example is severe acute respiratory syndrome–associated coronavirus in humans, in which the primary transmission event is believed to be the result of close human contact with civet cats in China. Once the infection was successful, it spread rapidly throughout the human population by direct contact (25).
The location of a host-pathogen system within the continuum will be determined by characteristics of both host populations and the pathogen. For instance, the pathogen's transmission mode will greatly determine its likelihood of encountering new hosts (26). Parasites transmitted by close contact may have limited exposure to multiple species and thus transmission modes that decouple host-to-host contact (i.e., waterborne or soilborne transmission) will increase the opportunity for between-species transmission. Evidence from wild primates and humans shows that pathogens with direct contact transmission are associated with high host specificity (1,3). Therefore, host-pathogen systems should segregate along the f12 axis according to their transmission mode.
Furthermore, the evolutionary potential of a pathogen will affect its ability to infect a new host (2,27). Pathogens in taxa with high mutation rates, antigenic diversity, and short generation times may rapidly adapt to new hosts (28,29), and recent evidence suggests that RNA viruses are the most likely group to emerge in humans (26,30), possibly because of their high mutation rate (31). Thus, host-parasite systems may segregate along the f22 axis according to taxonomy. Similarly, the phylogenetic relationship between the reservoir and target host will have consequences for disease emergence; viruses are less likely to jump to new hosts as the phylogenetic distance between hosts increases (32).
However, host-pathogen systems are not static, and a community may move across the continuum either because of ecologic or evolutionary shifts of the host or pathogen (27). In particular, anthropogenic changes, such as environmental exploitation and the introduction of domestic animals into previously uninhabited areas, may increase exposure to the pathogen and drive such transitions. For instance, although transmission of SIV from chimpanzees to humans may have occurred on a number of distinct occasions (6), these spillovers remained isolated. Only through various anthropogenic changes, including urbanization (increasing S2) and increased global travel (increasing β22) did the HIV pandemic take off in the 20th century.
In addition, pathogen evolution may greatly affect the likelihood of disease emergence by increasing the pathogen's basic reproductive ratio (R0) (18,26). For example, avian influenza has emerged several times in human populations since 1997. Typically, limited human-to-human transmission exists (β22 ≈ 0), so that although the avian reservoir (I1) and susceptible human populations (S2) are high, outbreaks are rare and isolated (i.e., occupying region 1 of the continuum). Only through recombination between strains and acquisition of human-specific respiratory epithelium receptors (thereby increasing β22) could the virus evolve sufficient transmissibility to be sustained in the human population, which poses the greatest risk for pandemics (33). These genetic changes could shift avian flu from being a spillover to becoming a true multihost parasite, which would have serious implications for human health.
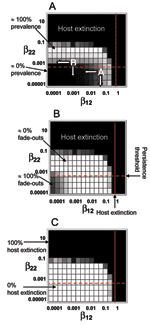
Theoretical and empiric evidence suggest that pathogens harbored by reservoir host populations are of particular concern because they can drive target hosts to extinction (34). Therefore, we must investigate population dynamic properties of different regions of the continuum and regions that pose the greatest risk for a target host. In a deterministic model, the invasion and persistence thresholds are the same and are determined by the pathogen's basic reproductive ratio (R0); if R0>1, an initial infection can both become established and persist. As shown by Dobson (15), R0 for a pathogen in an asymmetric host community (with no back-transmission from the target host to the reservoir) is dominated by the largest within-species transmission term, which implies that infection dynamics in the 2 host populations are largely independent; once between-species transmission has occurred, infection in H2 is driven solely by within-H2 transmission. However, in the stochastic reality of the natural world, an established infection may fade out, and reinfection from H1 could occur in the future (19). Therefore, we developed a stochastic analog of the above deterministic model to explore dynamics of the community-epidemiology continuum. The model was a discrete-time Monte Carlo simulation model, in which each event in model 1 (births, deaths, between- and within-species transmission) occurred probabilistically, and the next event was chosen at random based on those probabilities. The model was run 100 times for different combinations of within- and between-species transmission rates, and the infection status of the target host (H2) was measured as the mean prevalence over time, the proportion of time the pathogen was absent from H2 (the proportion of time the pathogen faded out), and the proportion of runs in which the pathogen drove the host to extinction. This stochastic model is appropriate for exploring the dynamics of emerging infectious diseases not captured by continuous-time deterministic models, in particular when exposure of a target host to a pathogen from a reservoir is likely to occur at discrete intervals (27).
As in the deterministic case, low between- and within-species transmission prevents the pathogen from persisting in the target host (prevalence ≈0, Figure 3A; proportion of time pathogen was absent ≈100%, Figure 3B). Increasing the exposure of H2 to the pathogen (i.e., increasing β12) leads to a gradual increase in both the prevalence of infection and the proportion of time the pathogen is present in H2. This increase applies even if within-H2 transmission is negligible (β22 → 0). Therefore, regular, high exposure to the pathogen from the reservoir can give the appearance of endemic infection, even if the pathogen cannot be sustained within the population (case 2: apparent multihost dynamics). Increasing the within-H2 transmission rate (β22) from very low levels has little impact on the prevalence of infection or the proportion of time H2 is infected. Eventually, however, a point is reached at which increasing β22 suddenly allows the long-term persistence of the pathogen in H2. At this point, the persistence threshold is reached and the pathogen becomes endemic in H2, regardless of input from H1. This threshold can be approximated from the deterministic model by setting β12 = 0 and solving for R0 = 1, which shows that β22 must be > (d + r)/K for the pathogen to persist in the absence of input from H1 (the horizontal line in Figure 3).
Increasing either between- or within-species transmission rates (β12 or β22) leads to a point when the host is driven to extinction (Figure 3C), which highlights the danger of an emerging infectious disease; even if H2 is a poor transmitter of the disease (β22 → 0), repeated exposure from H1 may be sufficient to drive the population to extinction. Analysis of the equivalent deterministic model (model 1) suggests that this threshold should be in the between-species transmission rate (β12) only (host extinction is not affected by β22) and is given by β12 > dr/(d – r) for H2 extinction to occur (shown by the vertical line in Figure 3). Thus, even if the probability that H2 will contract the pathogen is very low (β12 → 0), a single transmission event may spark an epidemic that completely decimates the population (region 3).
The correct classification of the different regions of the community-epidemiology continuum are of more than just semantic importance; quantifying the between- and within-species transmission rates and the location of a host-pathogen system within the continuum are vital to determine the appropriate control strategy. Haydon et al. (13) proposed 3 means of controlling infection in a target-reservoir system: 1) target control, which is aimed at controlling infection within the target population; 2) blocking tactics, to prevent transmission between the reservoir and target host population; and 3) reservoir control, which suppresses infection within the reservoir. These 3 control strategies correspond to reducing the within- and between-species transmission rates (β22, β12, and β11, respectively). The benefits of each approach will vary according to the relative contributions different transmission processes make to the overall prevalence in the new host (H2). Our stochastic model showed that high exposure to the pathogen from the reservoir host can give the appearance of endemic infection in the target host, even if it cannot sustain the pathogen alone. In this case, the optimal control strategy is completely different from that used against a true multihost pathogen endemic in the target host. For a host-pathogen system in region 2 of the continuum (apparent multihost dynamics), where between-species transmission rates are high but within-H2 transmission rates are low (point A in Figure 3A), the prevalence of infection in H2 may be very high, but mounting a target control program aimed at reducing within-H2 transmission is unlikely to be effective (the vertical arrow from point A in Figure 3A). However, blocking control, which would reduce transmission from the reservoir to the target host, may drastically reduce prevalence (the horizontal arrow from point A in Figure 3A). Conversely, similar levels of prevalence in H2 may be observed for a host-pathogen system located in region 4 of the continuum (point B in Figure 3A) but because of fundamentally different processes. In this case, blocking tactics aimed at preventing transmission from the reservoir to the target host will be ineffectual (horizontal arrow from point B in Figure 3A), but target control may prove highly effective (vertical arrow from point B in Figure 3A). Therefore, establishing the initial location of a novel host-pathogen system within the community-epidemiology continuum and understanding the within- and between-species transmission rates are essential for optimizing vaccination and culling strategies to lessen the impact of disease.
This report provides a conceptual framework to understand the ecologic characteristics of disease emergence based on between- and within-species transmission rates involving a potential disease reservoir population and a target host population. Using this framework, we outlined 4 possible cases of long-term disease dynamics in the target host and showed that these outcomes occupy different regions of a 2-dimensional continuum described by the net between- and within-species transmission rates. Furthermore, the development of the community-epidemiology framework allows us to clarify the wealth of terminology currently used to describe disease occurrence in host communities, based on an understanding of the underlying ecologic and epidemiologic processes. In particular, the much-overused terms reservoir and spillover can be seen to have explicit definitions, depending on whether the pathogen can be sustained within the target host population.
By explicitly considering how the ecologic and evolutionary characteristics of hosts and pathogens combine to affect the between- and within-species transmission rates, and the subsequent consequences for disease occurrence in a novel host, this framework highlights that current human diseases, domestic and wild animal diseases, and the threats of emerging infectious diseases can be understood by a quantitative framework of the underlying transmission processes. Given that most parasites can infect multiple host species and the recent surge of emerging infectious diseases in wildlife and human populations, understanding the dynamics of disease persistence in novel hosts has never been more important.
Dr Fenton is a National Environment Research Council research fellow at the University of Liverpool. His research interests include the dynamics of host-parasite systems, emerging infectious diseases, and the evolution of parasite life-history strategies.
Dr Pedersen is a postdoctoral researcher in the Department of Biology at the University of Virginia. Her research interests include the ecology of wildlife diseases and multihost, multiparasite community dynamics.
Acknowledgments
We thank S. Altizer for her enthusiasm about undertaking this project and helpful comments on the manuscript. We also thank J. Antonovics and M. Hood for comments on earlier drafts. This work was initiated at a workshop at Penn State University on the ecology and evolution of infectious diseases (Hudfest '03).
A.F. was funded by a fellowship from the National Environment Research Council.
References
- Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–9. DOIPubMedGoogle Scholar
- Cleaveland SC, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:991–9. DOIPubMedGoogle Scholar
- Pedersen AB, Altizer S, Poss M, Cunningham AA, Nunn CL. Patterns of host specificity and transmission among parasites of wild primates. Int J Parasitol. 2005;35:647–57. DOIPubMedGoogle Scholar
- Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science. 2000;287:443–9. DOIPubMedGoogle Scholar
- Roelke-Parker ME, Munson L, Packer C, Kock R, Cleaveland S, Carpenter M, A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature. 1996;379:441–5. DOIPubMedGoogle Scholar
- Hahn BH, Shaw GM, DeCock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–14. DOIPubMedGoogle Scholar
- Dobson A, Foufopolous J. Emerging infectious pathogens of wildlife. Philos Trans R Soc Lond B Biol Sci. 2001;356:1001–12. DOIPubMedGoogle Scholar
- Williams ES, Yuill T, Artois M, Fischer J, Haigh SA. Emerging infectious diseases in wildlife. Rev Sci Tech. 2002;21:139–57.PubMedGoogle Scholar
- Bowers RG, Begon M. A host-host-pathogen model with free-living infective stages, applicable to microbial pest control. J Theor Biol. 1991;148:305–29. DOIPubMedGoogle Scholar
- Begon M, Bowers RG. Host-host- pathogen models and microbial pest control: the effect of host self regulation. J Theor Biol. 1994;169:275–87. DOIPubMedGoogle Scholar
- Begon M, Bowers RG. Beyond host-pathogen dynamics. In: Grenfell BT, Dobson AP, editors. Ecology of infectious disease in natural populations. Cambridge (UK): Cambridge University Press; 1995. p. 478–509.
- Greenman JV, Hudson PJ. Parasite-mediated and direct competition in a two-host shared macroparasite system. Theor Popul Biol. 2000;57:13–34. DOIPubMedGoogle Scholar
- Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis. 2002;8:1468–73.PubMedGoogle Scholar
- Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM. Parasite establishment in host communities. Ecol Lett. 2003;6:837–42. DOIGoogle Scholar
- Dobson A. Population dynamics of pathogens with multiple host species. Am Nat. 2004;164:S64–78. DOIPubMedGoogle Scholar
- Ashford RW. When is a reservoir not a reservoir? Emerg Infect Dis. 2003;9:1495–6.PubMedGoogle Scholar
- Holt RD, Pickering J. Infectious disease and species coexistence: a model of Lotka-Volterra form. Am Nat. 1985;126:196–211. DOIGoogle Scholar
- Davis S, Begon M, de Bruyn, Ageyev VS , Klassovskiy NL , Pole SB , Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–8. DOIPubMedGoogle Scholar
- Gog J, Woodroffe R, Swinton J. Disease in endangered metapopulations: the importance of alternative hosts. Proc Biol Sci. 2002;269:671–6. DOIPubMedGoogle Scholar
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–29. DOIPubMedGoogle Scholar
- Rhodes CJ, Atkinson RPD, Anderson RM, Macdonald DW. Rabies in Zimbabwe: reservoir dogs and the implications for disease control. Philos Trans R Soc Lond B Biol Sci. 1998;353:999–1010. DOIPubMedGoogle Scholar
- Dobson A, Meagher M. The population dynamics of brucellosis in the Yellowstone National Park. Ecology. 1996;77:1026–36. DOIGoogle Scholar
- Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–41. DOIPubMedGoogle Scholar
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–8. DOIPubMedGoogle Scholar
- Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001;292:1109–12. DOIPubMedGoogle Scholar
- Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–61. DOIPubMedGoogle Scholar
- Whitlock MC. The red queen beats the jack-of-all-trades: the limitations on the evolution of phenotypic plasticity and niche breadth. Am Nat. 1996;148:S65–77. DOIGoogle Scholar
- Gupta S, Ferguson N, Anderson R. Chaos, persistence, and evolution of strain structure in antigenically diverse infectious agents. Science. 1998;280:912–5. DOIPubMedGoogle Scholar
- Holmes EC, Rambaut A. Viral evolution and the emergence of SARS coronavirus. Philos Trans R Soc Lond B Biol Sci. 2004;359:1059–65. DOIPubMedGoogle Scholar
- Drake JW, Charlesworth B, Charlesworth D, Crow JF. Rates of spontaneous mutation. Genetics. 1998;148:1667–86.PubMedGoogle Scholar
- DeFilippis VR, Villarreal LP. An introduction to the evolutionary ecology of viruses. In: Hurst CJ, editor. Viral ecology. San Diego (CA): Academic Press; 2000. p. 126–208.
- Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–22. DOIPubMedGoogle Scholar
- de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2004;7:117–26. DOIGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 11, Number 12—December 2005
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Andy Fenton, School of Biological Sciences, Biosciences Building, Crown Street, University of Liverpool, Liverpool L69 7ZB, UK; fax: 44-151-795-4408
Top