Volume 11, Number 2—February 2005
Research
Spotted Fever Group and Typhus Group Rickettsioses in Humans, South Korea
Cite This Article
Citation for Media
Abstract
The presence of the nucleic acid of the spotted fever group (SPG) and typhus group (TG) rickettsiae was investigated in 200 serum specimens seropositive for SFG rickettsiae by multiplex-nested polymerase chain reaction with primers derived from the rickettsial outer membrane protein B gene. The DNA of SFG, TG, or both rickettsiae was amplified in the 24 serum specimens, and sequence analysis showed Rickettsia conorii, R. japonica, and R. felis in the specimens. R. conorii and R. typhi were found in 7 serum specimens, which indicated the possibility of dual infection in these patients. These findings suggest that several kinds of rickettsial diseases, including boutonneuse fever, rickettsialpox, R. felis infection, and Japanese spotted fever, as well as scrub typhus and murine typhus, are occurring in Korea.
Human rickettsioses, known to occur in Korea, include mainly scrub typhus, murine typhus, and epidemic typhus. Scrub typhus, caused by Orientia tsutsugamushi, a major rickettsial disease in Korea, is transmitted through the bites of mite larvae. An earlier study by Choi and colleagues reported that 34.3% of febrile hospital patients in autumn were seropositive for the disease (1). Rickettsia typhi, transmitted by the fleas of various rodents, causes murine typhus, which is a milder form of typhus than human typhus (2). The first patient with murine typhus in Korea was reported in 1959. Two cases of murine typhus confirmed by culture were reported since 1988 (3,4), and now >200 cases of murine typhus are presumed to occur annually in South Korea. Epidemic typhus is caused by R. prowazekii and is transmitted by the body louse (5). The disease is fatal in 10% to 30% of patients, depending on underlying diseases and the nutritional state of the host (2). The disease appeared after the end of the Korean War. Since 1951, however, no other cases have been reported in Korea (6).
Spotted fever group (SFG) rickettsioses are associated with arthropods, such as ticks, mites, and fleas (2). SFG comprises several divergent lineages: the R. rickettsii group, R. japonica, R. montana, the R. massiliae group, R. helvetica, R. felis, and the R. akari group (2). Recently, the nucleic acids of R. japonica and R. rickettsii were found in Haemaphysalis longicornis in Korea (7). A previous seroepidemiologic study demonstrated that SFG rickettsioses were highly likely in Korea (8). No clinical human case of SFG rickettsioses, however, has been reported in Korea until now.
In this study, to check whether SFG rickettsioses were present in humans, serum specimens from patients with acute febrile disease were studied by using molecular sequence–based identification techniques. We report the presence of the rompB gene of SFG rickettsiae, similar to R. akari, R. conorii, R. japonica, and R. felis, in serum specimens from Korean patients with acute febrile disease. The nucleic acids of both R. conorii and R. typhi were found to coexist in 7 serum specimens. This study presents the first molecular evidence of SFG rickettsioses in humans.
Rickettsial Strains
The following strains were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA): R. typhi Wilmington (VR-144), R. prowazekii Breinl (VR-142), R. akari MK (VR-148), R. japonica YH (VR-1363), R. conorii Indian Tick Typhus (VR-597), and R. sibirica 246 (VR-151). These rickettsial agents were propagated in Vero (CRL-1586) or L929 (CCL-1) cell monolayers.
Serum Samples and Serologic Testing
The serum specimens analyzed in this study were obtained from South Korean patients with acute febrile illness from 1993 to 1999. The specimens were submitted to the Institute of Endemic Disease at Seoul National University’s Medical Research Center for laboratory diagnosis for scrub typhus, leptospirosis, and hemorrhagic fever with renal syndrome caused by hantavirus. Some of the serum specimens were used for the nucleic acid detection study of SFG rickettsial agents. The rationale for selecting the samples for polymerase chain reaction (PCR) analysis included the presence of immunoglobulin (Ig) M antibodies with titers from 1:40 to 1:160 against any of the tested antigens in the samples. Serologic testing was performed by indirect immunofluorescence assay (IFA) with a panel of 4 SFG rickettsial antigens, R. japonica, R. akari, R. conorii, and R. sibirica, as previously described (8).
Oligonucleotide Primers
The oligonucleotide primers used for priming the PCRs are shown in Table 1. The primers were developed on the basis of the rompB gene sequences of R. conorii strain Seven (GenBank accession no. AF123721), and the citrate synthase (gltA) gene sequence of R. prowazekii (GenBank accession no. M17149) was synthesized. The selection of the primers was based on the “primer 3” program (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi/), to obtain the optimal Tm and GC content and to avoid hairpin loop structures. The selected sequences were analyzed through the BLAST program (http://www.ncbi.hlm.hig.gov/BLAST).
Detection of rompB Gene in Human Sera
DNA for PCR analysis was extracted from 200 μL of serum samples by using QIAamp Blood Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. SFG and typhus group (TG) rickettsia rompB gene in human sera were detected with multiplex nested PCR. The primary amplification of the specimen was performed in a final reaction volume of 50 μL. The reaction mixture contained 5 μL of prepared DNA sample, 20 pmol of rompB outer forward primer (OF) and outer reverse primer (OR), 200 μM of deoxynucleoside triphosphate mixture (dNTP, Takara, Otsu, Japan), 1 x PCR buffer, 1.25 U Taq polymerase (Takara EX Taq, Takara), and distilled water. First, PCR reactions were incubated at 95°C for 5 min, subjected to 35 cycles of 95°C for 15 s, 54°C for 15 s, and 72°C for 30 s, and final extension at 72°C for 3 min in a GeneAmp PCR system 9600 (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). After this, 2 μL of the amplified product was again amplified in a nested fashion with inner primer sets (rompB SFG IF, rompB SFG/TG IR, and rompB TG IF). The nested PCR reaction mixture contained 10 pmol of each primer in a PCR premixture tube (AccuPower PCR PreMix, Bioneer Corp., Daejon, Korea) that contained 1 U of Taq DNA polymerase, 250 μmol/L each of dNTP, 50 mmol/L of Tris-HCl (pH 8.3), 40 mmol/L of KCl, 1.5 mmol/L of MgCl2, and gel loading dye. The volume was then adjusted to 20 μL with distilled water. Nested PCR reactions were incubated at 95°C for 5 min, subjected to 35 cycles of 95°C for 15 s, 56°C for 15 s, and 72°C for 30 s, and final extension at 72°C for 3 min. PCR amplification of the gltA gene of SFG and TG rickettsiae was performed by using the oligonucleotide pairs RpCS.877p and RpCS.1,258n for the primary PCR amplification and RpCS.896p and RpCS.1,233n for the secondary amplification. The primary PCR cycling condition consisted of incubation at 95°C for 5 min, then 35 cycles each of 15 s at 95°C, 15 s at 54°C, and 30 s at 72°C, followed by a final extension cycle of 3 min at 72°C. The nested PCR cycling condition consisted of incubation at 95°C for 5 min, then 35 cycles each of 15 s at 95°C, 15 s at 54°C, and 30 s at 72°C, followed by a final extension cycle of 3 min at 72°C. To avoid cross-contamination, 3 separate rooms with entirely separate equipment and solutions were used. Thus, the handling and treatment of samples and the addition of a template, the handling of DNA-free PCR reagents, and the post-PCR work were strictly separated. Aerosol-resistant tips (Axigen Scientific, Inc., Union City, CA, USA) were used for the handling of all reagents in the PCR study. The amplification products were visualized by electrophoresis on a 1.5% agarose gel stained with ethidium bromide (0.5 μg/mL) and using 1 x TAE migration buffer (pH 8.0; 40 mM Tris-acetate, 1 mmol/L EDTA).
Restriction Fragment Length Polymorphism (RFLP) Analysis
The PCR products were purified by using an AccuPrep PCR purification kit (Bioneer Corp.), according to the manufacturer’s instructions. Restriction endonuclease digestions were performed with 10 μL of amplified products by using AluI (New England Biolabs, Beverly, MA, USA). The digested DNA was resolved by electrophoresis through a 10% polyacrylamide gel at 100 V for 4 h in a 1 x TBE buffer (pH 8.0; 90 mmol/L Tris-borate, 2 mmol/L EDTA), and was visualized after staining with ethidium bromide.
Cloning, Sequencing, and Analysis of Nucleotide
All positive PCR products were cloned by using pGEM-T Easy Vector System I (Promega). Verifying whether the clones contained inserts was accomplished by digestion of plasmid DNA with EcoRI (New England Biolabs) and separation in 1.5% agarose gels. Plasmids containing DNA inserts were sequenced for both strands by using Big Dye Terminator Sequence Kit and ABI Prism 377 Automated DNA Sequencer (Perkin-Elmer Applied Biosystems), according to the manufacturer’s protocol. The obtained sequences, except for the primer regions, were aligned with the corresponding sequences of other rickettsiae deposited in the GenBank database to identify known sequences with a high degree of similarity using multisequence alignment programs, the Phydit software (10), and the MegAlign software package (Windows version 3.12e; DNASTAR, DYNASTAR Inc., Madison, WI, USA). Phylogenetic trees were generated by using the neighbor-joining algorithms and the Jukes and Cantor matrix. Bootstrap analysis was performed to investigate the stability of the trees obtained through the neighbor-joining method. The percentages of similarity were determined using the FASTA network service (European Bioinformatics Institute Fasta Service; available from http://www.ebi.ac.uk/fasta).
Nucleotide Sequence Accession Numbers Used
GenBank accession numbers of the rompB gene sequences used for sequence comparisons are AB003681 for R. japonica, AF123705 for R. aeschlimannii, AF123706 for R. africae, AF123707 for R. akari, AF123708 for Astrakhan rickettsia strain A-167, AF123709 for R. australis, AF123711 for R. honei strain RB, AF123712 for Israeli tick typhus rickettsia, AF123714 for R. massiliae, AF123715 R. mongolotimonae, AF123716 for R. montanensis, AF123717 for R. parkeri, AF123719 for R. rhipicephali, AF123721 for R. conorii strain Seven, AF123722 for R. sibirica, AF123723 for R. slovaca, AF123725 for R. helvetica, AF182279 for R. felis, AF211820 for R. prowazekii strain Florida, AF211821 for R. prowazekii strain Virginia, AF123718 for R. prowazekii, AF161079 for R. prowazekii, AF479763 for R. amblyommii strain WB-8-2 rompB pseudogene, AY260451 for R. heilongjiangensis, AY260452 for R. hulinensis, L04661 for R. typhi crystalline surface layer protein (slpT) gene, and X16353 for R. rickettsii. The GenBank accession number of the gltA gene sequence used for developing primers is M17149 for R. prowazekii.
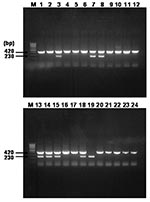
Multiplex Nested PCR Amplification of rompB Gene
Nested PCR assay, with primer pairs rompB OF and rompB OR in primary reactions and rompB SFG IF, rompB SFG/TG IR, and rompB TG IF in multiplex-nested reactions, was performed to identify the unknown rickettsial agents in the seropositive serum specimens and to differentiate between SFG and TG rickettsiae in terms of size. When the primers previously mentioned were used, the nested PCR assay generated ≈420 base pairs (bp) for SFG rickettsiae and about 230 bp for TG rickettsiae. The negative controls consistently failed to yield detectable PCR products, whereas the positive controls always gave the expected PCR products. Overall, 200 serum specimens from febrile patients from all areas of South Korea were tested. After the nested PCR was performed, the expected rompB gene products were obtained from 24 seropositive serum samples. Figure 1 shows the result of electrophoresis of 24 PCR-amplified samples. Of the 24 amplified products, 16 showed the electrophoretic pattern of 1 DNA band of ≈420 bp, which corresponded to SFG. The amplified size of only 1 sample was ≈230 bp for TG. The 7 other amplified products showed an electrophoretic pattern of 2 bands of ≈420 bp for SFG and 230 bp for TG. Therefore, the 23 amplified products corresponding to SFG rickettsial agents were named H1 product, while the 8 products corresponding to TG were named H2 product. The H1 products included H1 to H24 (except H19), while the H2 products were H3-2, H7-2, H8-2, H13-2, H14-2, H15-2, H18-2, and H19 (Figure 1).
RFLP Analysis and Sequencing Analysis
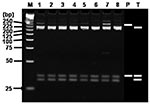
RFLP analysis of the 23 H1 products corresponding to SFG rickettsial agents using AluI demonstrated that the restriction patterns of 17 H1 products were identical with that of R. conorii, 2 with that of R. akari, 1 with that of R. japonica, and 3 with that of R. felis (Figure 2). RFLP analysis of the 8 H2 products corresponding to TG rickettsial agents by using AluI showed that the restriction patterns of all the H2 products were identical with that of R. typhi (Figure 3).
Sequencing Analysis
To identify the SFG and TG rickettsiae detected in human serum specimens, nucleotide sequences of the PCR-amplified products were determined and compared with partial rompB gene sequences of various rickettsial agents obtained from the GenBank database. Table 2 shows the similarity between the partial rompB gene sequences of various rickettsial agents and 6 of the sequenced H1 products (clones H1, H3, H5, H10, H20, and H22). Clones H1, H3, and H20 showed 100%, 99.72%, and 98.87% degrees of similarity to R. conorii, respectively. Clone H10 showed 100% similarity to R. japonica, and clone H5 showed 100% similarity to R. akari. In particular, clone H22 showed 99.44% similarity to R. felis. All the compared H1 products showed low levels of similarity (70.90%–74.01%) to the TG species. The clones that clustered partially with the rompB gene of R. conorii were differentiated in 3 groups by their levels of similarity: group 1 (12 H1 products with 100% similarity), group 2 (4 H1 products with 99.72% similarity), and group 3 (1 H1 product with 98.87% similarity). Clones H22, H23, and H24 clustered as the R. felis group. Table 3 shows the similarity between the partial rompB gene sequences of various rickettsial species and H2 product sequences. All H2 products showed low levels of similarity (67.05%–69.94%) to SFG rickettsial species, such as R. sibirica, R. akari, R. conorii, R. felis, and R. japonica. They also showed high levels of similarity (93.64%–100%) to TG rickettsial species, such as R. prowazekii and R. typhi. The H2 products’ levels of similarity to R. typhi ranged from 99.42% to 100%. A neighbor-joining analysis based on partial rompB gene sequences demonstrated that 17 H1 products formed a cluster with R. conorii, 2 with R. akari, 1 with R. japonica, and 3 with R. felis (data not shown). The analysis of the 8 H2 product sequences showed that the sequences of all H2 products formed a cluster with R. typhi and were separated from the SFG rickettsial strains (data not shown).
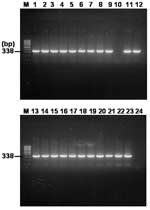
Nested PCR Amplification of gltA Gene
The results of the multiplex nested PCR of the rompB gene were confirmed by a second PCR assay with specific primer pairs RpCS.877p and RpCS.1,258 in primary reactions and RpCS.896p and RpCS.1,233 in nested reactions. The primer sets generated ≈338 bp for SFG and TG rickettsiae. The expected size of the gltA gene fragment was generated in 22 of 24 samples that were positive for the PCR detection of the rompB gene (Figure 4). All positive PCR products were cloned, and their sequences were determined. Since the PCR assay using primer sets for the amplification of the gltA gene could not discriminate between the SFG rickettsia and TG rickettsia by size difference, the sequences of 3 clones for each PCR product were determined. The results of the sequencing analysis for gltA-PCR amplifications were identical to those of the analysis of the rompB-PCR product (data not shown). Seven samples that were positive for both the rompB genes of R. conorii and R. typhi were also positive for both of their gltA genes (data not shown).
SFG and TG rickettsial infections occur worldwide and may cause serious diseases in humans. These pathogenic bacteria are transmitted to people by arthropod vectors, such as ticks, fleas, and lice. In this study, multiplex-nested PCR was conducted to detect and identify SFG and TG rickettsial antigens in patient sera with positive results from the serosurvey. The rompB gene domain II region, which is a highly conserved region of rompB, was targeted for PCR amplification for the specific detection of SFG and TG rickettsiae. Amplified DNA sequences were analyzed by using nucleotide-sequencing methods, and RFLP analysis was used to confirm the PCR results. The results indicated the presence of several SFG rickettsiae, R. conorii, R. akari, R. japonica, and R. felis, in the serum specimens. The results were also confirmed by a second PCR with specific primer pairs for the gltA gene and by sequence analysis of its DNA amplicons.
For the first time, SFG rickettsiae in human serum specimens in South Korea have been reported. R. akari is a member of the spotted fever group rickettsiae and is a causative agent of rickettsial pox, a disease transmitted by the bite of Allodermanyssus sanguineus, a mite ectoparasite of the domestic mouse (Mus muscularis) (2). The disease was first described in New York City in 1946. R. akari was isolated from the Korean vole in 1957. The previous seroepidemiologic study conducted by the authors on 3,401 patients with febrile disease indicated that the seropositive rate was 16.24% for the rickettsial antigen through IFA. R. conorii is an etiologic agent of the Mediterranean spotted fever or boutonneuse fever (2). Our previous study indicated that the seropositive rate was 14.34% for the antigen. R. japonica, the causative agent of Oriental spotted fever, was first isolated from a patient with febrile, exanthematous illness in Japan in 1985 (2). The disease is now endemic in the southwestern part of Japan, where >100 cases have been described (2). Previous studies showed the presence of nucleic acids of R. japonica and R. rickettsii in H. longicornis by PCR. Our seroepidemiologic study demonstrated that the seropositive rate was 19.9%. Although no clinical human case of SFG rickettsioses has been reported in Korea until now, this study’s findings strongly suggest the prevalence of SFG rickettsiosis in Korea.
R. felis is an emerging pathogen responsible for fleaborne spotted fever and had been considered a member of the TG rickettsiae based on its reactivity with anti–R. typhi antibodies. A genetic analysis of the 16S rRNA, citrate synthase, rompA, and rompB genes, however, placed R. felis as a member of SFG. R. felis has been reported in various countries, including the United States, Mexico, Brazil, Germany, and France (11,12). In Asia, the first case of R. felis infection was reported in 2003 (13). R. typhi was also among those detected in the SFG rickettsiae in the febrile disease patients’ sera. Fleas are also found to be vectors for R. typhi (2). Of major importance to the epidemiology of the rickettsioses caused by R. typhi and R. felis is the maintenance of both rickettsial agents in their hosts by transovarial transmission, and the fact that neither organism is lethal for fleas (14).
Finally, we report the presence of both R. conorii and R. typhi in serum from Korean patients. Sera from patients with SFG rickettsiosis have been reported to react with TG rickettsiae by using serologic analysis methods (15). The serum specimens from patients with TG rickettsiosis were also demonstrated to contain cross-reactive antibodies against SFG rickettsiae (15,16). In a previous study, approximately one third of specimens seropositive for antibodies against SFG rickettsiae had antibodies against TG rickettsiae (unpub. data). Therefore, the multiplex-nested PCR was designed to detect and differentiate SFG rickettsial agents from TG rickettsial agents in the patient serum specimens with positive results from the serosurvey with SFG rickettsial antigens. SFG rickettsiae and TG rickettsiae were differentiated in terms of the size of amplified products. PCR results also confirmed the RFLP and sequencing analysis. In sera taken from 7 patients, both SFG and TG rickettsial antigens were detected, which indicated dual infection. Previously, a case of dual infection with Ehrlichia chaffeensis and an SPG rickettsia was reported in a human patient. Cases of dual infection with Bartonella clarridgeiae and B. henselae in cats have also been reported, as well as infection with the 2 different genotypes of B. henselae (17,18). A recent report suggested that coinfection of R. felis with either B. clarridgeiae or B. quintana in fleas may cause dual infection in a human that comes in contact with flea feces (14). These reports support this study’s findings regarding the dual infection of SFG and TG rickettsiae in 7 patients. The differences between R. conorii and R. typhi vectors, however, still cannot be explained, and further studies are needed.
In conclusion, this study confirmed, by using PCR-based amplification methods, that several SFG rickettsiae, R. conorii, R. akari, R. japonica, and R. felis, existed in the sera of Korean patients with febrile episodes. Our findings indicate that SFG rickettsiae, including R. felis, should be used in serologic tests on Korean patients suspected of having rickettsiosis. TG rickettsiae existed in 8 patients, and 7 of them were also infected with R. conorii. The evidence of double infection is expected to help describe the cross-reactivity between the patient sera of SFG rickettsioses and TG rickettsioses.
Dr. Choi is a postdoctoral fellow in the Department of Microbiology College of Medicine Konkuk University, Choongcheongbuk-do, Korea. This work is part of her doctoral thesis. Her researches focus on the serologic and molecular epidemiology of various rickettsial diseases and the development of diagnostic tools.
Acknowledgment
This study was supported by a grant from the Korea Health 21 Research and Development Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6-01GM01-0004). This study was conducted in the Department of Microbiology, College of Medicine, Konkuk University.
References
- Choi MS, Park SK, Chang WJ, Huh MS, Kim HR, Han TH, A seroepidemiological survey on the scrub typhus in Korea, 1994. J Korean Soc Microbiol. 1994;30:593–602.
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719.PubMedGoogle Scholar
- Kim YW, Cho MK, Min CH, Yoon CS. Isolation and characterization of Rickettsia typhi from patients in Korea. J Korean Soc Microbiol. 1988;23:265–75.
- Song JW, Baek LJ, Lee YJ, Song KJ, Han SH. Seroepidemiologic analysis of acute rebrile illness from Korea in 1996. J Korean Soc Virol. 1998;28:377–82.
- Gross L. How Charles Nicolle of the Pasteur Institute discovered that epidemic typhus is transmitted by lice: reminiscence from my years at the Pasteur Institute in Paris. Proc Natl Acad Sci U S A. 1996;93:10539–40. DOIPubMedGoogle Scholar
- Chung HY. Suspected human infectious diseases in Korea; Rickettsial infections. Korean J Infect Dis. 1985;18:93–7.
- Lee JH, Park HS, Jung KD, Jang WJ, Koh SE, Kang SS, Identification of spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol. 2003;47:301–4.PubMedGoogle Scholar
- Jang WJ, Kim JH, Choi YJ, Jung KD, Kim YG, Lee SH, First serologic evidence of human spotted fever group rickettsiosis in Korea. J Clin Microbiol. 2004;42:2310–3. DOIPubMedGoogle Scholar
- Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol. 1997;47:252–61. DOIPubMedGoogle Scholar
- Chun J. Computer-assisted classification and identification of actinomycetes. [Ph.D. thesis]. Newcastle Tyne, United Kingdom: University of Newcastle upon Tyne; 1995.
- Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, Becerra-Carmona G, Walker DH. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–80. DOIPubMedGoogle Scholar
- Richter J, Fournier PE, Petridou J, Haussinger D, Raoult D. Rickettsia felis infection acquired in Europe and documented by polymerase chain reaction. Emerg Infect Dis. 2002;8:207–8. DOIPubMedGoogle Scholar
- Parola P, Miller RS, McDaneiel P, Telford SR III, Rolain JM, Wongsrichanalai C, Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis. 2003;9:592–5.PubMedGoogle Scholar
- Rolain JM, Franc M, Davousr B, Raoult D. Molecular detection of pathogenic Bartonella and Rickettsia in cat fleas from France. Emerg Infect Dis. 2003;9:338–42.PubMedGoogle Scholar
- Hechemy KE, Raoult D, Fox J, Han Y, Elliotte LB, Rawlings J. Cross-reaction of immune sera from patients with rickettsial disease. J Med Microbiol. 1989;29:199–202. DOIPubMedGoogle Scholar
- Uchiyama T, Zhao L, Yan Y, Uchida T. Cross-reacttivity of Rickettsia japonica and Rickettsia typhi demonstrated by immunofluorescence and Western immunoblotting. Microbiol Immunol. 1995;39:951–7.PubMedGoogle Scholar
- Gurfield AN, Boulouis HJ, Chomel BB, Kasten RW, Heller R, Bouillin C, Epidemiology of Bartonella infection in domestic cats in France. Vet Microbiol. 2001;80:185–98. DOIPubMedGoogle Scholar
- Abbott RC, Chomel BB, Kasten RW, Floyd-Hawkins KA, Kikuchi Y, Koehler JE, Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 2—February 2005
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ik-Sang Kim, Department of Microbiology and Immunology, Seoul National University College of Medicine and Institute of Endemic Disease, Seoul, 110-799, Republic of Korea; fax: 82-43-851-9329
Top