Volume 11, Number 3—March 2005
Research
Longitudinally Profiling Neutralizing Antibody Response to SARS Coronavirus with Pseudotypes
Figure 3
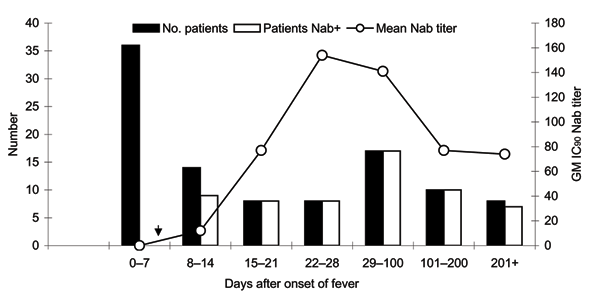
Figure 3. . Severe acute respiratory syndrome–associated coronavirus (SARS-CoV) neutralizing antibody–positive rate by time of blood sample collection (days after onset of fever). Black bars represent the number of patients tested for neutralizing antibodies (Nab). White bars represent the number of patients whose assayed samples were positive for neutralizing antibodies (Nab+). Samples are considered positive for Nab if the 90% neutralizing antibody titer determined by using murine leukemia virus (MLV) (SARS) pseudotypes is >10. Line plot with open white circles shows the geometric mean (GM) Nab titer within each time frame. IC90, 90% inhibitory concentration.
Page created: April 25, 2012
Page updated: April 25, 2012
Page reviewed: April 25, 2012
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.