Volume 11, Number 4—April 2005
Perspective
Emerging Infectious Diseases: a 10-Year Perspective from the National Institute of Allergy and Infectious Diseases
Figure 4
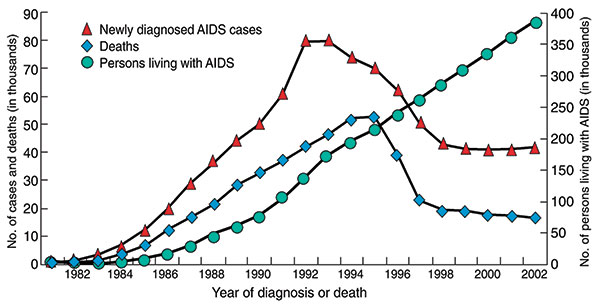
Figure 4. AIDS cases, AIDS deaths, and persons living with AIDS in the United States, 1981–2003. Over the past decade, the number of new AIDS cases and deaths due to AIDS has decreased, while the number of people living with the disease has increased, due in large part to improvements in diagnosis and treatment. Estimates adjusted for reporting delays. Source: CDC (8).
References
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–9. DOIPubMedGoogle Scholar
- Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675–85. DOIPubMedGoogle Scholar
- Institute of Medicine. Microbial threats to health: emergence, detection and response. Washington, DC: The Institute; 2003.
- Infectious Diseases Society of America. Bad bugs, no drugs as antibiotic discovery stagnates. A public health crisis brews. Washington, DC: The Society; 2004.
- Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–28.PubMedGoogle Scholar
- World Health Organization. The world health report 2004–changing history. Geneva: The Organization; 2004.
- UNAIDS. 2004 report on the global AIDS epidemic: 4th global report. [cited 2005 Feb 16]. Available from http://www.unaids.org/bangkok2004/report.html
- Centers for Disease Control and Prevention. HIV/AIDS surveillance report 2003. Atlanta: The Centers; 2003.
- Graham BS, Mascola JR. Lessons from failure—preparing for future HIV-1 vaccine efficacy trials. J Infect Dis. 2005;191:647–9. DOIPubMedGoogle Scholar
- Klausner RD, Fauci AS, Corey L, Nabel GJ, Gayle H, Berkley S, The need for a global HIV vaccine enterprise. Science. 2003;300:2036–9. DOIPubMedGoogle Scholar
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. DOIPubMedGoogle Scholar
- Holt R Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–49. DOIPubMedGoogle Scholar
- Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–3. DOIPubMedGoogle Scholar
- Johnson DJ, Ficock DA, Mungthin M, Lakshmanan V, Sidhu ABS, Bray PG, Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol Cell. 2004;15:867–77. DOIPubMedGoogle Scholar
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–20. DOIPubMedGoogle Scholar
- Jacobs-Lorena M. Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes. J Vector Borne Dis. 2003;40:73–7.PubMedGoogle Scholar
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomized controlled trial. Lancet. 2004;364:1411–20. DOIPubMedGoogle Scholar
- World Health Organization. Portfolio of malaria vaccines currently in development, October, 2004. [cited 2005 Feb 16]. Available from http://www.who.int/vaccine_research/documents/en/malaria_table.pdf
- Reed SG, Alderson MR, Dalemans W, Lobet Y, Skeiky YAW. Prospects for a better vaccine against tuberculosis. Tuberculosis (Edinb). 2003;83:213–9. DOIPubMedGoogle Scholar
- Butcher PD. Microarrays for Mycobacterium tuberculosis. Tuberculosis (Edinb). 2004;84:131–7. DOIPubMedGoogle Scholar
- Goguet de la Salmoniere YO, Kim CC, Tsolaki AG, Pym AS, Siegrist MS, Small PM. High-throughput method for detecting genomic-deletion polymorphisms. J Clin Microbiol. 2004;42:2913–8. DOIPubMedGoogle Scholar
- Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–28.PubMedGoogle Scholar
- Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71:1672–9. DOIPubMedGoogle Scholar
- National Institute of Allergy and Infectious Diseases. Tuberculosis: ancient enemy, present threat. [cited 2005 Feb 16]. Available from http://www2.niaid.nih.gov/newsroom/focuson/tb02/tb.htm
- Centers for Disease Control and Prevention. Influenza Web page. Available from http://www.cdc.gov/flu/
- World Health Organization. Influenza Web page. [cited 2005 Feb 16]. Available from http://www.who.int/topics/influenza/en/
- National Institute of Allergy and Infectious Diseases. Press releases, May 27, 2004 and August 17, 2004. [cited 2005 Feb 16]. Available from http://www2.niaid.nih.gov/Newsroom/Releases/news.htm?year=2004
- Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–103. DOIPubMedGoogle Scholar
- Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840–2. DOIPubMedGoogle Scholar
- Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–7. DOIPubMedGoogle Scholar
- Maassab HF, Francis T Jr, Davenport FM, Hennessy AV, Minuse E, Anderson G. Laboratory and clinical characteristics of attenuated strains of influenza virus. Bull World Health Organ. 1969;41:589–94.PubMedGoogle Scholar
- Harper SA, Fukuda K, Cox NJ. Using live, attenuated influenza vaccine for prevention and control of influenza: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2003;52(RR-13):1–8.PubMedGoogle Scholar
- Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. DOIPubMedGoogle Scholar
- Hall RA, Khromykh AA. West Nile virus vaccines. Expert Opin Biol Ther. 2004;4:1295–305. DOIPubMedGoogle Scholar
- Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7:759. DOIPubMedGoogle Scholar
- Borovsky D. Trypsin-modulating oostatic factor: a potential new larvicide for mosquito control. J Exp Biol. 2003;206:3869–75. DOIPubMedGoogle Scholar
- National Institute of Allergy and Infectious Diseases. NIAID research on severe acute respiratory syndrome (SARS). [cited 2005 Feb 16]. Available from http://www.niaid.nih.gov/factsheets/sars.htm
- Yang Z, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–4. DOIPubMedGoogle Scholar
- Bisht H, Roberts A, Vogel L, Bukreyev A, Collins PL, Murphy BR, Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A. 2004;101:6641–6. DOIPubMedGoogle Scholar
- Bukreyev A, Lamirande EW, Buchholz UJ, Vogel LN, Elkins WR, St Claire M, Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–7. DOIPubMedGoogle Scholar
- Subbarao K, McAuliffe J, Vogel L, Fahle G, Fischer S, Tatti K, Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–7. DOIPubMedGoogle Scholar
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–5. DOIPubMedGoogle Scholar
- National Institute of Allergy and Infectious Diseases. NIAID biodefense research. [cited 2005 Feb 16]. Available from http://www2.niaid.nih.gov/biodefense/
- National Center for Biotechnology Information. Entrez Genome. [cited 2005 Feb 16]. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Genome
- Earl PL Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–5. DOIPubMedGoogle Scholar
- Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101:4590–5. DOIPubMedGoogle Scholar
- Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZL, Roederer M, Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–4. DOIPubMedGoogle Scholar
- Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert Opin Biol Ther. 2004;4:965–73. DOIPubMedGoogle Scholar
Page created: May 23, 2011
Page updated: May 23, 2011
Page reviewed: May 23, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.