Volume 11, Number 7—July 2005
Letter
Third Borrelia Species in White-footed Mice
Cite This Article
Citation for Media
To the Editor: The white-footed mouse, Peromyscus leucopus, is a natural reservoir host of several pathogens, including Borrelia burgdorferi, an agent of Lyme borreliosis (LB) (1). B. burgdorferi spirochetes are transmitted in the mouse population by Ixodes scapularis ticks. This tick vector also bears B. miyamotoi, a sister species to the relapsing fever group of spirochetes (2,3). B. miyamotoi infects P. leucopus in the laboratory (2), but the role of this mouse as a reservoir was not known. Here we report that P. leucopus is a reservoir for B. miyamotoi in nature and, in addition, that this mouse is host for a third, hitherto unknown, species of Borrelia.
In a recent study of a 9-hectare site in a mixed hardwood forest in eastern Connecticut, we found that ≈35% of I. scapularis nymphs were infected with B. burgdorferi and ≈6% were infected with B. miyamotoi (4). For that study of a field vaccine we also collected blood from P. leucopus mice captured from June to early September of 2001. DNA was extracted from the blood and then subjected to quantitative polymerase chain reaction (PCR) assay for the presence of B. burgdorferi as described (4). In the present study, we analyzed the extracts of 556 blood samples from 298 mice from the nonvaccine control grids by a multiplex, quantitative real-time PCR for 16S rDNA that discriminated between B. burgdorferi and B. miyamotoi at the site (4). Sixty-nine (12%) of the samples were positive for B. burgdorferi and 36 (6%) were positive for B. miyamotoi; 5 (0.9%) of the samples were positive for both species. In infected mice, the mean number of B. miyamotoi cells per milliliter of blood was 251 (95% confidence limits of 126–631), 5-fold greater than that of B. burgdorferi at 50 cells/mL (40–63).
A standard PCR assay of the blood samples with primers for the 16S–23S rDNA intergenic spacer (IGS) was performed as described by Bunikis et al. (5); results suggested the presence of a third species of Borrelia among the blood samples of the mice. A uniquely sized amplicon of ≈350 bp was observed in the reactions of 6 of 100 samples that were positive for B. burgdorferi and or B. miyamotoi by 16S PCR, and of 2 of 31 randomly selected samples that were negative for both B. burgdorferi and B. miyamotoi (p = 0.3 by 2-sided exact chi-square test).
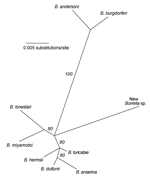
Samples with the 350-bp amplicon were further investigated by PCR assay with Borrelia genus-specific primers for the 16S rRNA gene (rDNA), as described by Barbour et al. (6). The resultant ≈830-bp PCR product from these samples was directly sequenced on a Beckman 3000CEQ automated sequencer (5). The 788-bp sequence was aligned with sequences of other Borrelia species representing the LB and relapsing fever clades, and phylogenetic analysis was conducted. The accompanying Figure shows that the new species clusters with the monophyletic relapsing fever group of species rather than with the LB group species. However, the new spirochete is distinct from all other known Borrelia spp. with an available 16S rDNA sequence in the GenBank database. Its partial 16S rDNA sequence differed by 3.3% to 4.2% from 9 LB group species and 2.4% to 3.4% from 15 relapsing fever group species. For comparison, intragroup sequence differences were ≤1.9%. On this basis, as well as the finding of partial IGS sequences (GenBank accession nos. AY668955 and AY668956) that were unique among all Borrelia spp. studied to date (3,5), we propose that this is a new species of Borrelia, provisionally named Borrelia davisii in honor of Gordon E. Davis for his contributions to Borrelia research and taxonomy.
While the new species was detected in 8 of 131 P. leucopus blood samples by using PCR for the IGS, the assays for this organism in the DNA extracts of 282 I. scapularis nymphs (4) from the same geographic site were uniformly negative (p = 0.0003, 2-sided Fisher exact test). This finding suggests that the new spirochete has another vector. The only other documented tick species that has been found feeding in small numbers on P. leucopus in Connecticut is Dermacentor variabilis (7). Holden et al. reported the presence of Borrelia in D. variabilis ticks in California by using PCR with genus-specific primers, but the species in these ticks was not identified by sequencing (8).
Although how B. miyamotoi and B. davisii affect the health of humans and other animals remain to be determined, our finding of 3 Borrelia species with overlapping life cycles in the same host in the same area shows that the ecology of Borrelia is more complex than was imagined. The presence of species other than B. burgdorferi in a major reservoir will have to be considered in future surveys and interventions.
Acknowledgments
We thank Hany Mattaous and Lili Sheibani for technical assistance and Jean Tsao, Klara Hanincova, and Durland Fish for discussions and ongoing collaborative studies.
This work was supported by grants to AGB (AI37248 from the National Institutes of Health) and to JB (Cooperative Agreement 919558-01 from the Centers for Disease Control and Prevention).
References
- Levine JF, Wilson ML, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–60.PubMedGoogle Scholar
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. DOIPubMedGoogle Scholar
- Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10:1661–4.PubMedGoogle Scholar
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–64. DOIPubMedGoogle Scholar
- Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–55. DOIPubMedGoogle Scholar
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–9. DOIPubMedGoogle Scholar
- Stafford KC III, Bladen VC, Magnarelli LA. Ticks (Acari: Ixodidae) infesting wild birds (Aves) and white-footed mice in Lyme, CT. J Med Entomol. 1995;32:453–66.PubMedGoogle Scholar
- Holden K, Boothby JT, Anand S, Massung RF. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J Med Entomol. 2003;40:534–9. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 11, Number 7—July 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jonas Bunikis, Department of Microbiology and Molecular Genetics, B240 Medical Sciences I, University of California Irvine, Irvine, CA 926974025, USA; fax: 949-824-6452
Top