Volume 31, Number 11—November 2025
Dispatch
Borrelia afzelii Hepatitis in Patient Treated with Venetoclax and Obinutuzumab, Switzerland
Cite This Article
Citation for Media
Abstract
We report Borrelia afzelii hepatitis in an immunosuppressed patient in Switzerland receiving anti-CD20 therapy and venetoclax. Diagnosis was made by metagenomic sequencing and PCR. This case underscores the need to consider Lyme borreliosis in unexplained hepatitis cases and highlights the value of molecular diagnostics in immunosuppressed patients when serologic test results are negative.
Lyme borreliosis (LB) presents a wide range of clinical manifestations across its stages. Early localized infection (stage 1) typically manifests as erythema migrans, whereas later stages involve systemic complications (1,2). In North America, Borrelia burgdorferi sensu stricto is the predominant causative agent (3). Possible manifestations of early dissemination are multiple erythema migrans, arthritis, or acute neuroborreliosis (1,2). In Europe, B. afzelii and B. garinii are more common causes (3); B. afzelii is the most frequent cause of erythema migrans, lymphocytoma, and acrodermatitis chronica atrophicans, and B. garinii primarily causes neuroborreliosis (1,2). Although mild hepatopathy occurs in up to 27% of LB cases in the United States (4) and in 14%–15% of cases in Europe (5), hepatic infection by Borrelia spp. is rare (6). We report a case of hepatic infection caused by B. afzelii in a patient in Switzerland with chronic lymphocytic leukemia (CLL) receiving venetoclax and obinutuzumab. Written informed consent for participation in this case report was obtained from the patient by the authors.
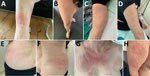
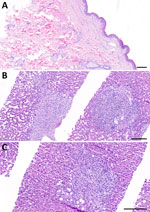
A 62-year-old white woman with CLL diagnosed in 2016 was managed with watchful waiting until late 2023, when biopsy-confirmed leukemia cutis developed on her right shoulder. In March 2024, she began a chemotherapy regimen of obinutuzumab, a novel anti-CD20 monoclonal antibody, and venetoclax. One week later, she reported nonpruritic erythematous rashes on her legs; the first appeared on the right ankle, and additional rashes spread to the right and left leg. Clinically, multiple circular erythematous exanthemas with a maximum diameter of 10 cm were present on both legs (Figure 1). After 2 weeks, the rash on the left leg had further expanded, exhibiting discrete central clearing (Figure 1). In addition to that progression, similar new exanthemas appeared on the trunk and both arms (Figure 1). A skin biopsy showed a mild superficial and deep lymphocytic perivascular dermatitis (Figure 2, panel A). Initial test results for Borrelia IgM were negative; borderline IgG elevation was noted (10.78 AU/mL [reference <10 AU/mL]) (Diasorin, https://int.diasorin.com). Results of immunoblot (Virotech, http://www.virotechdiagnostics.com) were positive only for variable major protein-like sequence, expressed. Topical steroids were initiated for suspected morphea.
One month later, the exanthemas showed slight improvement, except for those on the right arm and upper chest (Figure 1). At that time, the patient began experiencing persistent fever of temperatures exceeding 38.5°C and elevated C-reactive protein (84 mg/L [reference <10 mg/L]); leukocyte and transaminase levels were within reference ranges, but cholestatic parameters were mildly elevated (gamma-glutamyl transferase 55 U/L [reference 6–40 U/L]; alkaline phosphatase 317 U/L [reference 35–105 U/L]). Results of a whole-body computed tomography scan were unremarkable. A drug fever was suspected; venetoclax and obinutuzumab were discontinued in June 2024. Three weeks later, the patient was hospitalized for persistent fever, worsening cholestatic parameters (gamma-glutamyl transferase189 U/L, alkaline phosphatase 804 U/L), new hepatitis (aspartate transferase 140 U/L [reference 11–34 U/L], alanine transaminase 80 U/L [reference 8–41 U/L]), and elevated C-reactive protein (120 mg/L). Results of serologic testing and PCR were negative for hepatitis B, C, and E; Epstein-Barr virus; cytomegalovirus; herpes simplex virus; adenovirus; HIV; Bartonella henselae; Coxiella burnetii; Brucella spp.; Toxoplasma gondii; Schistosoma spp.; Leishmania spp.; and dimorphic fungi. Results of blood cultures, including those for mycobacteria, were negative, as was follow-up serologic testing for Borrelia. Liver ultrasound revealed discrete hepatomegaly with mildly mottled parenchyma; Fibroscan (Echosens, https://www.echosens.com/en-us) showed a slightly elevated stiffness (7.6 kPa [reference 2–7 kPa]). Positron emission tomography–computed tomography showed global hepatic hypermetabolism. Liver biopsy showed chronic cholestatic hepatitis with acute cholangitis but preserved lobular architecture (Figure 2, panels B, C). Immunohistochemistry results for cytomegalovirus and Epstein-Barr virus were negative; unfortunately, no sample for microbiology was taken. To further investigate the origin of hepatitis, metagenomic next-generation sequencing (mNGS) performed on the liver biopsy identified B. afzelii, which was confirmed by nested PCR (Appendix). Retrospective PCR analysis of the skin biopsy was also positive for B. afzelii, establishing the diagnosis of early disseminated LB with multiple erythema migrans and hepatitis.
The patient was treated with ceftriaxone (2 g/d for 3 wks) and fully recovered (Figure 3). Patient history revealed tick bites in the year before the onset of symptoms. The patient, who resides in a small village in a valley in Switzerland, had been treated for erythema migrans ≈20–30 years previously.
We documented a case of B. afzelii hepatitis in an immunosuppressed patient receiving anti-CD20 therapy and venetoclax. The case underscores the diagnostic challenge of LB in severely immunocompromised persons, in whom serologic testing might be unreliable and mNGS offers a valuable diagnostic tool.
We found only 6 cases of histologically confirmed Borrelia-associated hepatitis in the literature; of those, 2 cases were PCR-confirmed (B. garinii and B. burgdorferi s.s.) (7–12) (Appendix Table). Histology typically showed sinusoidal and portal inflammation, Kupffer cell hyperplasia, granulomatous hepatitis, or a combination of those; spirochetes were visualized in 2 cases. Clinically, most hepatitis manifested in fever and nonspecific symptoms. Abdominal pain was reported in 1 case and erythema migrans in another case.
The pathogenesis of hepatic injury in LB likely involves direct hepatic infiltration by spirochetes and immune-mediated damage (4). An in vitro study suggests that vascular adhesion and emigration might represent key strategies used by B. burgdorferi sensu lato to evade the intravascular innate immune response, enabling persistence in organs such as the liver (13). Subsequent inflammatory infiltration and Kupffer cell activation contribute to hepatic injury (14). In our case, although Warthin-Starry staining did not reveal spirochetes, its limited sensitivity and strong background staining do not exclude presence of spirochetes in the liver tissue. The pronounced granulomatous inflammation, together with elevated liver enzymes and hepatomegaly, strongly suggests local hepatic involvement rather than a passive inflammatory reaction to circulating pathogens. Although contamination from residual blood cannot be entirely ruled out, the minimal blood content in formalin-fixed, paraffin-embedded tissue makes this an unlikely explanation for the robust mNGS signal observed.
Furthermore, this case underscores the importance of promptly recognizing erythema migrans, which is primarily a clinical diagnosis. Early identification and treatment might have prevented dissemination and hepatic involvement, especially in immunocompromised patients. In this patient, initial IgG serologic testing was borderline positive, but later samples were negative, illustrating the limitations of serologic testing in immunosuppressed patients. Maraspin et al. (15) reported that only 28.6% (2 of 7) of rituximab-treated patients with erythema migrans tested positive for Borrelia in serologic testing, compared with 62.7%–68.6% in immunocompetent persons. Moreover, Borrelia dissemination in those patients was more frequent; isolation rates of Borrelia spp. from skin (80%) and blood (40%) were higher than in immunocompetent patients (55%–63% for skin and 2% for blood). Those findings reinforce the need for biopsy-based molecular diagnostics when LB is suspected in immunocompromised patients with negative serology.
In conclusion, this case highlights the importance of considering LB in the differential diagnosis of acute hepatitis, particularly in LB-endemic regions and in patients with epidemiologic risk factors. In immunocompromised patients, negative results of serologic testing do not exclude infection, and invasive diagnostic approaches such as biopsy with molecular testing (PCR, mNGS) might be essential for accurate diagnosis and timely treatment. Noninvasive diagnostic tools such as plasma microbial cell-free DNA sequencing should also be considered.
Dr. Capoferri is a specialist in infectious diseases and internal medicine. His research interests include bacterial infections, infections in immunosuppressed patients, and clinical epidemiology.
Acknowledgments
We thank all healthcare personnel and laboratory staff involved in the patient’s care.
The nonhuman fraction of the next-generation sequencing data will be available in the European Nucleotide Archive upon publication.
G.C. and M.W. provided care for the patient and wrote the manuscript. R.B. was involved in clinical management of the patient. P.M.K. provided microbiological advice and performed microbiological testing. B.H. and K.D.M. performed histological analysis, PCR, and whole-genome sequencing. All authors reviewed and approved the manuscript.
References
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. DOIPubMedGoogle Scholar
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73. DOIPubMedGoogle Scholar
- Marques AR, Strle F, Wormser GP. Comparison of Lyme Disease in the United States and Europe. Emerg Infect Dis. 2021;27:2017–24. DOIPubMedGoogle Scholar
- Zaidi SA, Singer C. Gastrointestinal and hepatic manifestations of tickborne diseases in the United States. Clin Infect Dis. 2002;34:1206–12. DOIPubMedGoogle Scholar
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (Southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523–39. DOIPubMedGoogle Scholar
- Srivastava B, Gimson A. Hepatic changes in systemic infection. Best Pract Res Clin Gastroenterol. 2013;27:485–95. DOIPubMedGoogle Scholar
- Chavanet P, Pillon D, Lancon JP, Waldner-Combernoux A, Maringe E, Portier H. Granulomatous hepatitis associated with Lyme disease. Lancet. 1987;2:623–4. DOIPubMedGoogle Scholar
- Goellner MH, Agger WA, Burgess JH, Duray PH. Hepatitis due to recurrent Lyme disease. Ann Intern Med. 1988;108:707–8. DOIPubMedGoogle Scholar
- Dadamessi I, Brazier F, Smaïl A, Delcenserie R, Dupas JL, Capron JP. [Hepatic disorders related to Lyme disease. Study of two cases and a review of the literature] [in French]. Gastroenterol Clin Biol. 2001;25:193–6.PubMedGoogle Scholar
- Zanchi AC, Gingold AR, Theise ND, Min AD. Necrotizing granulomatous hepatitis as an unusual manifestation of Lyme disease. Dig Dis Sci. 2007;52:2629–32. DOIPubMedGoogle Scholar
- Middelveen MJ, McClain SA, Bandoski C, Israel JR, Burke J, MacDonald AB, et al. Granulomatous hepatitis associated with chronic Borrelia burgdorferi infection: a case report. Research. 2014;1:875. DOIGoogle Scholar
- Duffau P, Korbi S, Guillotin V, Talagrand-Reboul E, Ménard A, Peuchant O. An unexpected case of Borrelia garinii liver infection. Ann Clin Microbiol Antimicrob. 2022;21:15. DOIPubMedGoogle Scholar
- Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. DOIPubMedGoogle Scholar
- Duray PH, Steere AC. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. DOIPubMedGoogle Scholar
- Maraspin V, Bogovič P, Rojko T, Ružić-Sabljić E, Strle F. Erythema migrans: course and outcome in patients treated with rituximab. Open Forum Infect Dis. 2019;6:ofz292. DOIGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: December 04, 2025
Table of Contents – Volume 31, Number 11—November 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Gioele Capoferri, Division of Infectious Diseases, University Hospital Basel, Petersgraben 4, Basel CH-4031, Switzerland
Top