Volume 11, Number 7—July 2005
Synopsis
SARS Vaccine Development
Figure 2
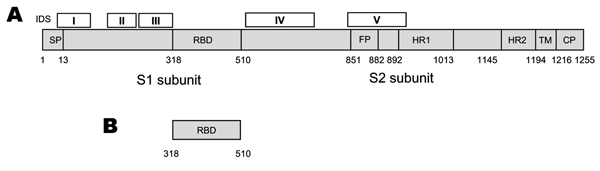
Figure 2. . Strategies for designing vaccines for severe acute respiratory syndrome (SARS) using A) spike (S) protein and B) fragments containing neutralizing epitopes. SP, signal peptide; RBD, receptor binding domain; FP, fusion peptide; HR, heptad repeat; TM, transmembrane domain; CP, cytoplasm domain. IDS, immunodominant sites I to V corresponding to the sequences of amino acid residues 9–71, 171–224, 271–318, 528–635, and 842–913, respectively. The residue numbers of each region correspond to their positions in the S protein of SARS–associated coronavirus (SARS-CoV) strain Tor2. RBD contains the major neutralizing epitopes in the S protein. The recombinant RBD may be used as an efficacious and safe vaccine for preventing infection by SARS-CoV strains with distinct genotypes.