Volume 12, Number 6—June 2006
Research
Norwalk Virus–specific Binding to Oyster Digestive Tissues
Cite This Article
Citation for Media
Abstract
The primary pathogens related to shellfishborne gastroenteritis outbreaks are noroviruses. These viruses show persistence in oysters, which suggests an active mechanism of virus concentration. We investigated whether Norwalk virus or viruslike particles bind specifically to oyster tissues after bioaccumulation or addition to tissue sections. Since noroviruses attach to carbohydrates of the histo-blood group family, tests using immunohistochemical analysis were performed to evaluate specific binding of virus or viruslike particles to oyster tissues through these ligands. Viral particles bind specifically to digestive ducts (midgut, main and secondary ducts, and tubules) by carbohydrate structures with a terminal N-acetylgalactosamine residue in an α linkage (same binding site used for recognition of human histo-blood group antigens). These data show that the oyster can selectively concentrate a human pathogen and that conventional depuration will not eliminate noroviruses from oyster tissue.
Twelve years ago, the question, "Should shellfish be purified before public consumption?" was asked in Lancet (1). Since then, new evidence of gastroenteritis outbreaks linked to shellfish consumption, even depurated shellfish, has been published, and raw or cooked oysters are the predominant bivalve mollusks involved (2–5). Regulations for Escherichia coli counts in shellfish-growing waters (United States) or shellfish meat (European Community) have failed to protect consumers because most shellfish-associated gastroenteritis outbreaks have a viral origin (4). Enteric viruses are different from enteric bacteria in terms of resistance to sewage treatment, persistence under unfavorable conditions such as occur in sea water, and transmission into the environment (6–8). Shellfish mollusks cultivated in coastal areas close to human activities can be contaminated by human sewage, which can spread >100 types of viruses (9). Viruses persist in shellfish for an extended period and can adversely affect public health; despite improvements, depuration does not eliminate viral particles (2,10–14).
Noroviruses are the most frequent cause of diarrhea outbreaks in all age groups (8,15). These viruses, which are commonly associated with foodborne and waterborne outbreaks, are resistant to sewage treatment and are present in high concentrations during the epidemic season (3,7,15). They are the primary pathogens associated with shellfishborne outbreaks worldwide (3,4). Oysters are rapidly contaminated, as shown by outbreaks linked to accidental input, and viruses then persist for up to several weeks (2,13,16). These data suggest that contamination by a passive process during mollusk filter feeding may be too simplistic an explanation. We tested whether oysters can actively capture noroviruses and determined the fate of prototype genogroup I Norwalk virus particles in bioaccumulation experiments. Since noroviruses can attach to carbohydrates of the ABH and Lewis histo-blood group family in humans, we also examined the possibility of a similar specific binding to oyster tissues through related carbohydrates (17,18).
Norwalk Virus Strain and Recombinant Viruslike Particles
Norwalk virus strain 8FIIa was purified from a stool sample of an infected volunteer and assayed by reverse transcription–polymerase chain reaction (RT-PCR) endpoint dilution (RT-PCR units) to determine the amount of viral RNA in the sample (19). Based on the equivalence of 1 RT-PCR unit to 30–40 genomic copies of RNA, the titer was estimated to be ≈3 × 1010 particles/mL (2).
Constructs containing open reading frames 2 and 3 were used to produce recombinant viruslike particles (VLPs) as described previously (20). Virus concentration, as calculated with the detergent compatible protein assay (Bio-Rad Laboratories, Hercules, CA, USA), was 2.11 × 1015 particles/mL. The number of particles in 1 μg of VLPs was calculated by multiplying the molecular mass of the major capsid protein VP1 (58 kDa) by the number of copies of the protein in a particle (180) and by a mass of 1 Da (1.66 × 10–24 g) (21).
Mutants in the P2 subdomain of VP1 were generated by Ala point substitution and used to produce recombinant VLPs His 329 (H329A), Asn 331 (N331A), and Trp 375 (W375A) (22). Concentrations of these 3 mutant VLPs were adjusted to 5.78 × 1014 particles/mL.
Oysters
All experiments were performed with Crassostrea gigas from a clean area (Class A, European Regulations), with no viral contamination, as tested after viral concentration, RNA extraction, and RT-PCR (2). Two batches of oysters collected in March and October 2004 were used to prepare tissue sections.
Bioaccumulation of Norwalk Virus and Recombinant VLPs
Norwalk virus (5 × 108 particles) or recombinant VLPs (1012 or 109 particles) were added to 500 mL clean sea water and homogenized for 5 min. Three oysters were added to the sea water and incubated at room temperature for 12 or 24 h. Sea water was continuously aerated to maintain adequate oxygen levels. A negative control was used under similar conditions but without the addition of Norwalk virus or recombinant VLPs.
Immunohistochemical Analysis
Oysters (uncontaminated or after bioaccumulation) were shucked, and the flesh was fixed in 10% formaldehyde for 48 hours. The digestive gland was then dissected, embedded in paraffin, and sliced into thin sections (5 μm). After preparation of tissue sections, sections from uncontaminated oysters were covered with 4 × 109 particles of recombinant wild-type VLPs, 3 × 109 particles of Norwalk virus, or 2 × 108 particles of mutant VLPs, incubated overnight at 4°C, and washed 3 times (5 min per wash) in phosphate-buffered saline (PBS) at room temperature (23). VLPs bound to oyster tissue or virus trapped after the bioaccumulation experiments was detected by using a rabbit polyclonal antibody to recombinant VLP as previously described (23). Negative controls included sections from uncontaminated samples not exposed to Norwalk virus or recombinant VLPs and virus-exposed sections without antibody. Immunoreactivity, which was detected by microscopic analysis, was graded as strong (intense brown-red staining), weak (pale brown-red staining), or negative (no staining).
Inhibition of Recombinant VLP Binding to Tissue Sections
Treatment with periodate was performed as previously described (23). VLPs were incubated overnight on slides, and the immunohistochemical detection was conducted. Saliva samples from 18 persons with secretor type O, 14 with secretor type A, 4 with secretor type B, and 5 nonsecretors were tested. Phenotyping was performed by enzyme-linked immunosorbent assay, and secretor phenotype was confirmed by genotyping as previously described (17). For the inhibition assay, VLPs were mixed with saliva samples diluted 1:100 for 90 min at room temperature, placed on shellfish tissues, and incubated overnight at 4°C. Positive (without treatment) and negative (without recombinant VLPs) samples were included in this assay.
Binding and Inhibition by Specific Lectins and Carbohydrate-specific Antibodies
Three biotinylated lectins, derived from Helix pomatia (HPA), Dolichos biflorus (DBA), and Ulex europaeus (UEA-1) (Biovalley SA, Marne la Vallée, France), were used for analysis of binding to tissues and inhibition of VLP binding. UEA-1 recognizes the H type 2 trisaccharide and shows some cross-reactivity with Ley. HPA and DBA recognize α-linked N-acetylgalactosamine residues. For the binding assay, lectins were applied to shellfish tissues for 30 min at different concentrations (50, 20, 10, 5, and 1 μ>g/mL).
Monoclonal antibodies were used for tissue staining. The antibodies used were anti-A (3-3A), which recognizes all A epitopes; anti-A types 3/4 (III-2A3, III-2A18, and III-2A24); anti-A type 2 (III-2A5); anti-H/Leb (LM-137); anti-H type 2 (19-OLE); anti-Ley (12-4LE); and anti-H type 1. The primary antibodies were serially diluted, incubated with tissues overnight at 4°C, and subjected to standard immunohistochemical analysis (23). Negative (without lectin or primary antibody) and positive (known rat or human tissue sections) controls were also tested. For inhibition assays, antibodies or lectins were applied to shellfish tissues for 1 h at 37°C or 1 h at 4°C, respectively, at concentrations 10-fold higher than the lowest dilution that showed binding to the oyster tissues, incubated with VLPs, and analyzed as described above.
Binding of Virus in Oysters after Bioaccumulation
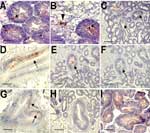
Oysters were immersed in sea water seeded with Norwalk virus at a final concentration of 106 particles/mL or recombinant VLPs at final concentrations of 2 × 109 and 2 × 106 particles/mL. After incubation for 12 h, virus was detected in digestive diverticula. Immunostaining showed particles in the lumen of tubules and ducts or in phagocytes between epithelial cells and in the surrounding connective tissue (Figure, panels A and B). No difference was observed for the 2 recombinant VLP concentrations used. After incubation for 24 h in sea water at final concentrations of 5 × 108 and 5 × 109 particles/mL for Norwalk virus and recombinant VLPs, respectively, similar results were obtained with particles binding to digestive ducts and isolated cells in connective tissue (data not shown).
Binding of Virus in Oyster Digestive Tissues
The binding of virus or recombinant VLPs was tested directly on oyster gut sections. Both recombinant VLPs and native virions bound to the midgut, the main and secondary ducts of the digestive diverticula, and tubules, although labeling of tubules was weaker (Figure, panels C, D, and E). Binding to connective tissue was not observed. Staining was not observed in negative controls.
Recombinant VLPs can attach to human digestive epithelial cells by recognizing carbohydrate structures. To determine if attachment to oyster digestive cells was also carbohydrate dependent, tissues sections were treated with 10 mmol/L sodium periodate before adding the VLPs. A reduction in VLP binding occurred, which suggested that, similar to the case with human tissue, binding to oyster tissue involves carbohydrates (Figure, panels E and F).
Inhibition of Virus Binding to Oyster Digestive Cells by Human Saliva
Since attachment of recombinant VLPs to human tissue sections involves carbohydrates of the ABH, secretor, and Lewis histo-blood group family, which are present on human salivary mucins, we evaluated the ability of saliva of different ABO and secretor phenotypes to block the binding of VLPs to shellfish tissues. Complete inhibition of binding was observed after pretreatment of the recombinant VLPs with type A secretor saliva, and binding was markedly reduced after incubation with type O secretor saliva. However, after incubation with type B secretor saliva or nonsecretor saliva, binding was as strong as that observed in positive controls (Table 1). All samples in subgroups of saliva showed similar patterns.
Binding of Mutant Recombinant VLPs
Our results suggested that attachment of VLPs to oyster tissue involves carbohydrate binding sites that overlap the site that attaches to human digestive cells. This site has been mapped to a restricted area of the viral capsid P2 domain. Mutations in key residues in this domain inactivate the binding activity to histo-blood group structures. To confirm that the binding to oyster tissue involved the same binding site, VLPs with point mutations were tested. Among 3 mutants, only mutant N331A binds to tissues similar to parental recombinant VLP (Figure, panel G). The 2 other mutants, H329A and W375A, did not bind to tissues (Figure, panel H).
Inhibition of VLP Binding by Antibodies to Carbohydrate and Lectins
Antibodies to carbohydrates on cells in the human digestive tract can inhibit binding of recombinant VLPs to human tissue (18). Primary antibodies that recognize various determinants of the histo-blood group family were evaluated for binding to oyster digestive tract and inhibition of VLP binding. Antibodies that recognize all types of A determinants (3-3A) or those that are restricted to A type 3/4 determinants bound strongly to shellfish tissues (Table 2). Anti-H type 1 BG-4 labeled oyster digestive cells intracellularly and also labeled connective tissue. Antibody LM137, which recognizes all H determinants and Leb tetrasaccharide, also labeled connective tissue and digestive epithelial cells intracellularly. However, staining had a more punctiform appearance. Despite recognition of oyster ligands, in inhibition assays none of these antibodies inhibited binding of VLPs to oyster tissues. Antibodies directed against H type 2 (19-OLE,), Ley (12-4LE), or A type 2 epitopes (III-2A-5) showed little staining of shellfish tissues and when positive, staining did not correspond to cells to which VLPs bind. These results indicate that oyster tissues have carbohydrates that resemble human histo-blood group antigens, but structures recognized by VLPs were not identical to those recognized by these antibodies.
Lectins have been used to determine the distribution of carbohydrate residues in tissue. Since lectins have a broader reactivity than monoclonal antibodies, we used lectins to identify recombinant VLP-specific carbohydrate ligand in oyster tissue. UEA-1 bound to shellfish tissues, but only at high concentration (50 μg/mL), whereas 2 other lectins, DBA and HPA, bound at a lower concentration (1 μg/mL) (Table 3). Tissue and cellular distribution of staining overlapped with that of the recombinant VLPs (Figure, panel I). When these lectins were used in an inhibition assay, only HPA had an inhibitory effect on binding of recombinant VLPs to shellfish tissues. Complete inhibition was observed at a concentration of 25 μg/mL (Table 3). This inhibitory effect was reproducible and observed on tissues from different shellfish, indicating that attachment of recombinant VLPs to oysters involves carbohydrate structures with a terminal N-acetylgalactosamine residue in an α linkage.
Virus-mediated disease can be transmitted when contaminated shellfish are eaten. Oysters are believed to act as filters or ionic traps, passively concentrating particles. A simple depuration process should be sufficient to rid oysters of virus, as observed for bacteria (12). However, long-term virus persistence in shellfish is a serious public health issue, and depuration or relaying is known to be inefficient (10,12,14). After bioaccumulation, only 7% of Norwalk virus is depurated, compared to a 95% reduction in bacterial levels (13). Virus is located mainly in pancreatic tissue (digestive diverticula), and various mechanisms have been suggested to explain differences between oyster species regarding virus accumulation, such as mechanical entrapment or ionic bonding (10,13,24,25).
Our data demonstrate specific binding of viral particles from a genogroup I norovirus to the oyster digestive tract and suggest a specific mechanism for concentration of virus particles. We tested recombinant VLPs of prototype genogroup I Norwalk virus. VLPs are stable in the marine environment and the disinfection processes (14,26). Bioaccumulation and tissue-binding experiments showed no difference between native Norwalk virus and VLPs, which confirmed that VLPs are good surrogates of infectious virions.
Different results were seen between bioaccumulation experiments and particle binding to tissues sections. After bioaccumulation, some viral particles were detected in phagocytes in either epithelium or connective tissue. This finding could reflect elimination of virus during digestion. The time required for food to pass through the entire shellfish intestinal tract varies from 90 to 150 min. We do not know whether immunoreactive material detected in phagocytes corresponds to particle degradation and digestion or if particles can escape digestion. Binding to main ducts may provide a mechanism for viral particles to avoid entering the digestive system and being degraded. Specific attachment of virus to oyster cells and capture by phagocytes may explain why depuration in oysters is not an effective mechanism for eliminating virus.
Virus accumulation in oysters may depend on factors such as water temperature, mucus production, glycogen content of connective tissue, or gonadal development (25). In our study, no difference was seen in binding location between samples collected in March or October, although studies during other seasons are warranted.
Human susceptibility factors for norovirus infections that depend upon carbohydrates of the ABH, secretor, and Lewis histo-blood group family have been observed (17,27). We showed that recognition of oyster digestive epithelial cells by recombinant VLPs also involves carbohydrates. The ability of human saliva to inhibit attachment of VLPs to oyster tissue in a histo-blood group–dependent manner indicates involvement of the histo-blood group binding site and the viral P2 subdomain (18).
Similar to human histo-blood group structures, mutant VLPs showed that an alanine substitution at positions H329A and W375A prevented binding to oyster tissue. However, a mutant with a substitution at position N331A did not affect binding. This binding specificity identified the amino acids required for binding and further confirmed the similarity with the mechanism of recognition of human tissues (22). However, inhibition experiments using antibodies to carbohydrates showed that ligands on oyster tissues are not identical to those on human tissues. Since attachment of recombinant VLPs was blocked by lectin from H. pomatia, which recognizes α-linked N-acetylgalactosamine terminal residues of glycans, the oyster ligands are similar to those of histo-blood group A. The DBA lectin did not inhibit attachment of recombinant VLPs, which indicates that it does not bind oligosaccharide structures recognized by VLPs and HPA. Although DBA binds to N-acetylgalactosamine residues similar to HPA, the 2 lectins have different carbohydrate specificities that depend on the subjacent sugar residues (28). Thus, Norwalk virus binds to oyster tissues through an A-like carbohydrate structure recognized by HPA at a binding site also used for attachment to carbohydrate on human epithelial cells.
Genogroup I and II strains of norovirus show various binding patterns with different carbohydrate structures of the histo-blood group family, which suggests coevolution of this group of viruses and their host or carrier vector. The ability of Norwalk virus to bind to oysters tissues at the same binding site as that used to bind to human tissues suggests a possible coevolution mechanism involving the oyster as an intermediary vector. This mechanism would favor selection of some viruses, such as Norwalk virus, over other viruses that are unable to bind to the oyster and would not be transmittable by this intermediary host. Epidemiologic data suggest a predominance of genogroup I strains in oyster-related gastroenteritis outbreaks, whereas genogroup II strains are predominant in other food-related outbreaks (2,3,5,8,15,16). To clearly address this point, binding of other norovirus strains needs to be evaluated because VLP-carbohydrate binding patterns differ, and binding should also be evaluated with other shellfish species (18,27).
As knowledge increases in understanding the binding of these viruses in humans, we will likely further understand more about their behavior in shellfish. This knowledge may help identify species that could be less sensitive to contamination. Since the oyster can actively and specifically bind a human pathogen, this knowledge has practical consequences because conventional depuration cannot eliminate noroviruses from oyster tissues.
Dr Le Guyader is a molecular biologist at the Institut Français de Recherche pour l'Exploitation de la Mer in Nantes. Her research interests include norovirus released by humans and shellfish contamination.
Acknowledgments
We thank Jezabel Rocher for technical assistance and R. Fraser, J. Bara, and Signet Pathology Systems for providing monoclonal antibodies.
This work was supported in part by the European Commission, QLRT-1999-0634, and the Institut National de la Santé et de la Recherche Médicale.
References
- Bosch A, Xavier Abad F, Gajardo R, Pinto RM. Should shellfish be purified before public consumption? Lancet. 1994;344:8928. DOIPubMedGoogle Scholar
- Le Guyader FS, Neil FH, Dubois E, Bon F, Loisy F, Kohli E, A semi-quantitative approach to estimate Norwalk-like virus contamination of oysters implicated in an outbreak. Int J Food Microbiol. 2003;87:107–12. DOIPubMedGoogle Scholar
- Kageyama T, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Kojima S, Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to Norovirus in Japan. J Clin Microbiol. 2004;42:2988–95. DOIPubMedGoogle Scholar
- Butt AA, Aldridge KE, Sanders CV. Infections related to the ingestion of seafood. Part I: viral and bacterial infections. Lancet Infect Dis. 2004;4:201–12. DOIPubMedGoogle Scholar
- Gallimore CL, Cheesbrough JS, Lamden K, Bingham C, Gray JJ. Multiple norovirus genotypes characterised from an oyster-associated outbreak of gastroenteritis. Int J Food Microbiol. 2005;103:323–30. DOIPubMedGoogle Scholar
- Griffin DW, Donaldson KA, Paul JH, Rose JB. Pathogenic human viruses in coastal waters. Clin Microbiol Rev. 2003;16:129–43. DOIPubMedGoogle Scholar
- Duizer E, Bijkerk P, Rockx B, de Groot A, Twisk F, Koopmans M. Inactivation of caliciviruses. Appl Environ Microbiol. 2004;70:4538–43. DOIPubMedGoogle Scholar
- Lopman BA, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A, Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–8. DOIPubMedGoogle Scholar
- Metcalf TG, Melnick JL, Estes MK. Environmental microbiology: from detection of virus in sewage and water by isolation to identification by molecular biology—a trip of over 50 years. Annu Rev Microbiol. 1995;49:461–87. DOIPubMedGoogle Scholar
- Heller D, Gill ON, Raynham E, Kirkland T, Zadick PM, Stanwell-Smith R. An outbreak of gastrointestinal illness associated with consumption of raw depurated oysters. BMJ. 1986;292:1726–7. DOIPubMedGoogle Scholar
- Richards GP. Microbial purification of shellfish: a review of depuration and relaying. J Food Prot. 1988;51:218–51.
- Schwab KJ, Neill FH, Estes MK, Metcalf TG, Atmar RL. Distribution of Norwalk virus within shellfish following bioaccumulation and subsequent depuration by detection using RT-PCR. J Food Prot. 1998;61:1674–80.PubMedGoogle Scholar
- Loisy F, Atmar RL, Le Saux J-C, Cohen J, Caprais M-P, Pommepuy M, Rotavirus virus like particles as surrogates to evaluate virus persistence in shellfish. Appl Environ Microbiol. 2005;71:6049–53. DOIPubMedGoogle Scholar
- Widdowson MA, Sulka A, Bulens SN, Beard RS, Chaves SS, Hammond R, Norovirus and foodborne disease, United States, 1991–2000. Emerg Infect Dis. 2005;11:95–102.PubMedGoogle Scholar
- Le Guyader FS, Neill FH, Estes MK, Monroe SS, Ando T, Atmar RL. Detection and analysis of a small round structured virus strain in oysters implicated in an outbreak of acute gastroenteritis. Appl Environ Microbiol. 1996;62:4268–72.PubMedGoogle Scholar
- Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–53. DOIPubMedGoogle Scholar
- Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–93. DOIPubMedGoogle Scholar
- Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. DOIPubMedGoogle Scholar
- Bertolotti-Ciarlet A, Crawford SE, Hutson AM, Estes MK. The 3´ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J Virol. 2003;77:11603–15. DOIPubMedGoogle Scholar
- White LJ, Ball JM, Hardy ME, Tanaka TN, Kitamoto N, Estes MK. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J Virol. 1996;70:6589–97.PubMedGoogle Scholar
- Hutson AM, Chakravarty S, Atmar RL, Prasad BVV, Estes MK. Identifying the binding site on Norwalk virus for cell-surface carbohydrates. In: Abstracts of Digestive Disease Week and the 106th Annual Meeting of the American Gastroenterological Association; Chicago, IL; 2005 May 14–19 [Abstract 393]. Gastroenterology. 2005;128(Suppl 2):A-56.
- Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, Ruiz-Palacios G, Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–77. DOIPubMedGoogle Scholar
- Di Girolamo R, Liston J, Matches J. Ionic binding, the mechanism of viral uptake by shellfish mucus. Appl Environ Microbiol. 1977;33:19–25.PubMedGoogle Scholar
- Burkhardt W, Calci KR. Selective accumulation may account for shellfish-associated viral illness. Appl Environ Microbiol. 2000;66:1375–8. DOIPubMedGoogle Scholar
- Caballero S, Abad FX, Loisy F, Le Guyader FS, Cohen R, Pinto RM, Rotavirus virus-like particles as surrogates in environmental persistence and inactivation studies. Appl Environ Microbiol. 2004;70:3904–9. DOIPubMedGoogle Scholar
- Hutson AM, Atmar RL, Estes MK. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol. 2004;12:279–87. DOIPubMedGoogle Scholar
- Piller V, Piller F, Cartron JP. Comparison of the carbohydrate-binding specificities of seven N-actyl-D-galactosamine-recognizing lectins. Eur J Biochem. 1990;31:461–6. DOIGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 12, Number 6—June 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Françoise S. Le Guyader, Laboratoire de Microbiologie, Institut Français de Recherche pour l’Exploitation de la Mer, BP 21105, 44311 Nantes CEDEX 03, France
Top