Volume 13, Number 2—February 2007
Research
Community-associated Methicillin-resistant Staphylococcus aureus Isolates and Healthcare-Associated Infections1
Cite This Article
Citation for Media
Abstract
We noted a marked increase in healthcare-associated (HA) methicillin-resistant Staphylococcus aureus (MRSA) infections caused by isolates phenotypically consistent with community-associated (CA)-MRSA strains. To study this trend, we retrospectively examined all HA-MRSA isolates from patients in our institution during 1999–2004. An isolate was considered an SCCmecIV phenotype if it had antimicrobial drug susceptibilities consistent with typical CA-MRSA isolates. Our phenotypic definition was validated in a limited subset of isolates by SCCmec genotype, pulsed-field gel electrophoresis, and multilocus sequence typing. Among 352 patients with HA-MRSA isolates, SCCmecIV phenotype increased from 17% in 1999 to 56% in 2003 (p<0.0001). Antimicrobial drug-susceptibility phenotype and genotype were consistent in 21 (91%) of 23 isolates. In a multivariate model, the SCCmec type IV phenotype was independently associated with wound culture source, later year of collection, and MRSA isolated earlier during hospitalization. In conclusion, MRSA isolates phenotypically similar to CA strains have become the predominant isolates associated with HA-MRSA in our hospital.
Methicillin-resistant Staphylococcus aureus (MRSA) is the most frequently identified antimicrobial drug–resistant pathogen in US hospitals (1). The epidemiology of infections caused by MRSA is rapidly changing. In the past 10 years, infections caused by this organism have emerged in the community. The 2 MRSA clones in the United States most closely associated with community outbreaks, USA400 (MW2 strain, ST1 lineage) and USA300, often contain pvl genes and, more frequently, have been associated with skin and soft tissue infections (2,3). Outbreaks of community-associated (CA)–MRSA infections have been reported in correctional facilities, among athletic teams, among military recruits, in newborn nurseries, and among men who have sex with men (4–7). CA-MRSA infections now appear to be endemic in many urban regions and cause most CA–S. aureus infections (5,6,8–10).
CA-MRSA isolates were first recognized by distinct resistance profiles of antimicrobial drugs that lacked resistance to older antimicrobial drugs (11–13). Several groups have noted these distinct susceptibility patterns appearing in isolates from hospitalized patients. Denis et al. noted that since 1995, MRSA isolates in Belgian hospitals were losing resistance to older antimicrobial drugs such as gentamicin and clindamycin (14). A Spanish hospital experienced a decrease in gentamicin-resistant MRSA isolates (from 97% in 1998 to 20% in 2002) and a simultaneous increase in MRSA isolates carrying the SCCmec type IV cassette (from 0% prevalence in 2000 to 23% prevalence in 2002) (15). A French group noted a similar finding in their hospitals over an 11-year period and found a correlation between isolates that contained SCCmec type IV and susceptibility profiles to ≥3 antimicrobial drugs (16). However, these investigations did not distinguish between cultures obtained from patients hospitalized with CA infection and those with hospital-associated (HA) infections. Thus, it is unclear whether these trends in decreased antimicrobial drug resistance and increased number of MRSA isolates that contained SCCmec type IV were due to increased hospitalization of patients with CA-MRSA infections or to an increased prevalence of isolates containing SCCmec type IV among HA-MRSA isolates.
Some MRSA strains associated with CA infection have been noted to cause HA infections. Outbreaks of HA infections caused by isolates containing SCCmec type IV have been reported from Australia and the United States. Affected populations have included postpartum women and patients undergoing prosthetic joint replacement (17–19). Another recent report demonstrated that CA strains had emerged as a substantial cause of HA bloodstream infections (20). However, these reports are anecdotal, and data examining temporal trends are lacking.
At our institution, which is located in an area in which CA-MRSA infections are endemic, we have noted a large increase in HA infections caused by MRSA isolates that, by assessment of antibiotic susceptibility patterns, appear to carry the SCCmec type IV element (e.g., susceptible to gentamicin, clindamycin, and trimethoprim-sulfamethoxazole) (6,10,21). The aim of this study was to quantify this trend over a 6-year period.
Population
To find patients with HA-MRSA infections, we identified all cultures obtained >72 hours after hospitalization that grew MRSA, from January 1, 1999, through December 31, 2004, at Harbor-UCLA Medical Center, a tertiary-care, urban, county hospital in Los Angeles County. At this hospital, surveillance cultures for MRSA colonization are not routinely performed; therefore, cultures positive for MRSA are likely to reflect infection rather than colonization. For a given patient, we examined only data from the first positive culture and excluded patients who had positive cultures both ≥72 hours and <72 hours after admission. If a patient had been hospitalized more than once during the study period, only data from the first hospitalization were retained. A standardized instrument was used to abstract data from the medical record of each patient. Information obtained included demographics, admission date and time, hospital location, antimicrobial drug susceptibility of the MRSA isolate, and time, date, and source of the MRSA culture.
We obtained only MRSA blood isolates for molecular typing because the clinical microbiology laboratory discards all other types of isolates after identification is complete. In vitro susceptibilities were reported as minimal inhibitory concentrations and performed with the VITEK system (bioMérieux, Durham, NC, USA), according to the protocols of the Clinical and Laboratory Standards Institute (CLSI). The investigation protocol was reviewed and approved by the Institutional Review Board of Harbor-UCLA Medical Center.
Molecular Characterization of Strains
Molecular typing was performed at the University of Chicago by investigators who were blinded to the clinical details and antibiograms of the isolates.
SCCmec Typing
PCR was performed to detect mecA by using the primer pair mecAF/mecAR (22). SCCmec elements were distinguished by the molecular architecture of the ccr and mecA complexes (21,23,24). PCR typing of SCCmec types I–IV was performed under the conditions previously described (24,25). SCCmec type II (ccrAB complex type 2 and mec complex class A), SCCmec type III (ccrAB complex type 3 and mec complex class A), and SCCmec type IV (ccrAB complex type 2 and mec complex class B) were assigned according to published criteria (25). PCR primers used to detect mecI (primers mI3/mI4), the mecR1 membrane spanning region (MS) (primers mcR3/mcR4), and the mecR1 penicillin-binding region (PB) (primers mcR1/mcR5) were originally reported by Suzuki et al. (26). Screening for ccrAB complex types 1, 2, and 3 (ccrAB 1, 2 and 3) was accomplished with a multiplex PCR assay that uses a mixture of 4 primers designed by Ito et al., consisting of a common forward primer (β2) and reverse primers, α2, α3, and α4 specific for ccrAB complexes 1, 2, and 3. Thermocycler conditions used have been described (27). The presence of the ccrAB gene complex allotype 4 (ccr complex 4) was assessed in a separate reaction that used the primer pair ccrA4F and ccrB4R (27). Screening for the ccrC gene (ccr complex 5) was performed with a forward primer (γF) in combination with the reverse primer γR described by Ito et al. (28). Prototype strains used for SCCmec typing were NCTC10442 (SCCmec I), N315 (SCCmec II), 85/2082 (SCCmec III), MW2 (SCCmec IV), and WIS (SCCmec V). The control strain used for detection of ccrAB4 was S. epidermidis strain ATCC 12228, which contains ccrAB4 in the non–mec-containing SCCcomposite island (24).
MLST
MLST was performed by PCR amplification and sequencing of 7 housekeeping genes by using the primer pairs designed by Enright et al (29). Denville Taq-Pro Complete (Denville Scientific, Metuchen, NJ, USA) or the Taq DNA Polymerase (Promega, Madison, WI, USA) was used for the PCR reactions. PCR products were evaluated on an agarose gel and purified by using Millipore 96-well Montage (Billerica, MA, USA) plates according to manufacturer’s instructions. The purified templates were sequenced at the University of Chicago Core Sequencing Facility and evaluated with the use of Vector NTI software (Invitrogen, Carlsbad, CA, USA). Each sequence was submitted to the MLST database website (www.mlst.net) for assignment of the allelic profile and sequence type (ST).
Screening for pvl Genes
Isolates were screened for the lukF-PV and lukS-PV genes encoding the components of the PVL toxin by PCR amplification of a 433-bp product that includes a portion of both the lukS-PV and lukF-PV ORFs by using the primer pair luk-PV-1/ luk-PV-2 (final concentration 0.2 μM) designed by Lina et al. (30). The thermocycler conditions have been described (27).
Case Definition and Data Analysis
A standardized definition of CA-MRSA infection was created by the Centers for Disease Control and Prevention (CDC) Active Bacterial Core Surveillance sites (31). Using this definition, we defined HA-MRSA infections as those MRSA infections that did not meet the definition of CA-MRSA infections. Specifically, we defined an MRSA isolate as HA associated if the original entry criteria of hospitalization for >72 hours before culture acquisition was met and if in the year before the present hospitalization, the patient had had any 1 of the following: hospitalization, surgery, residency in a long-term care facility, and hemodialysis or peritoneal dialysis, or at the present admission had indwelling percutaneous devices or catheters. A CA infection was defined as a culture-confirmed MRSA infection without any of the above criteria. However, if the patient did not meet any of the above criteria, had an infection at the time of admission, and the culture of the infection on admission was taken ≥72 hours after admission, then the infection was considered CA. An example of this situation would be a deep tissue infection microbiologically diagnosed from a surgical biopsy specimen 4 days after the patient’s admission.
To validate our definition of HA-associated infection, we reviewed 105 (30%) randomly selected charts of the patients with MRSA infections identified ≥72 hours after hospitalization. The purpose of this validation was to confirm that these cultures did not reflect CA infections that were diagnosed late (>72 hours) in the hospital course. Of note, in the CDC definition, an infection is considered HA if it occurs >48 hours after admission. Yet, we chose >72 hours as a cut-off to more conservatively capture HA infections, i.e., to minimize the miscategorization of CA infections as HA infections.
We then defined MRSA strains as having the SCCmec type IV phenotype if the isolates were resistant to oxacillin and susceptible to gentamicin, clindamycin, and trimethoprim-sulfamethoxazole. All other isolates were considered to be phenotypically non–SCCmec type IV.
Characteristics were compared between patients infected with the non–SCCmec type IV phenotype isolates and those infected with SCCmec type IV phenotype isolates by using a χ2 or t test, as appropriate. No adjustments were made for multiple comparisons. Temporal trends in the proportion of the SCCmec type IV phenotype were compared with the Cochran-Armitage test of trends. A multivariate analysis that predicted phenotypically SCCmec type IV isolates was performed by using an unconditional logistic regression model and a backward model selection method. A p value of <0.05 was defined as statistically significant. Data analysis was done with SAS software version 8.2 (SAS Institute Inc., Cary, NC, USA).
Population Characteristics
We identified 352 patients who had HA-MRSA cultures; 229 (65%) were men, and the median age was 50 years (mean 49.5 years). In the subset of medical records reviewed for validation of HA or CA status, none of the patients’ infections (0/105) fit our definition of a CA infection. The SCCmec type IV phenotype was identified in 128 (36%) of these 352 patients. Compared with the non–SCCmec type IV phenotype, patients with the SCCmec type IV phenotype were younger (median age 48 vs. 54 years, p = 0.02) and had the defining culture taken earlier in the hospitalization (median 8 vs. 15 days, p = 0.01). Finding an isolate with the SCCmec type IV phenotype was more likely if the culture source was from a wound, blood, or source other than sputum (odds ratio [OR] 2.9, 95% confidence interval [CI] 1.7–5.0, p<0.0001; OR 2.6, 95% CI 1.2–5.7, p = 0.02; and OR 1.2, 95% CI 0.6–2.3, p = 0.69) (Table 1).
Validation of the SCCmec Phenotype Definition
Of the 352 cultures, 35 were recovered from blood and were potentially available for genetic analysis. We were able to subculture 24 of the blood isolates. We could not perform SCCmec typing on 1 of the 24 growing isolates. The 23 remaining isolates were representative of each year of the 6-year period except 1999, when no isolates could be recovered.
Twelve isolates carried the SCCmec type IV element, and 9 also carried the pvl genes (Table 2). Eleven isolates carried the SCCmec type II element; none carried pvl. The clinical definition of the SCCmec IV phenotype was fulfilled by 11 (92%) of the 12 isolates that carried the SCCmec IV element. The exception was an isolate that contained SCCmec IV that was resistant to gentamicin, clindamycin, and trimethoprim-sulfamethoxozole. The definition of the non-SCCmec IV phenotype was fulfilled by 10 (91%) of 11 isolates carrying the SCCmec II element. Phenotypic case definition of SCCmec type was highly correlated with the genotype confirmation of the SCCmec type phenotype (p<0.0001 by Fisher exact test).
Trend and Multivariate Analysis of the SCCmec type IV Phenotype
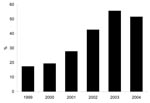
The proportion of MRSA isolates with the SCCmec type IV phenotype increased from 17% in 1999 to 56% in 2003 (p<0.0001, Figure). The proportion of isolates that were of the SCCmec type IV phenotype in 2004 (52%) was little changed from 2003 (Figure). In the multivariate model, independent predictors for having an SCCmec type IV phenotype isolate were wound source of culture (referent group was sputum source, OR 2.6, 95% CI 1.5–4.6, p = 0.001), culture obtained in less time after admission, (OR 0.88 per week, 95% CI 0.8–0.98, p = 0.02), and year of culture acquisition (p<0.0001) (Table 1).
In many urban centers worldwide, infections due to MRSA account for a large proportion of CA–S. aureus infections; in some communities MRSA accounts for more than half of CA–S. aureus infections (6,8–10,32). There have been reports of strains frequently associated with community outbreaks causing HA infections, but they have been mostly limited to case reports or case series (17–19). To our knowledge, ours is the first investigation quantifying the rise of MRSA isolates typical of CA disease to become the predominant strain of HA-MRSA (i.e., accounting for >50% of MRSA strains) within the hospital setting. Remarkably, at our institution the number of HA-MRSA isolates that have a CA phenotype, which previously was uncommon, now is >50%.
Our analysis found 3 significant risk factors for an SCCmec type IV phenotype MRSA culture. First, patients with MRSA cultures from a wound source were more likely to have the SCCmec type IV phenotype. This finding may be understandable, given that the most common clinical syndrome described with CA-MRSA infections has been skin and soft tissue infections (10,33). In addition, 75% of CA-MRSA isolates that were genotyped carried the pvl gene, which has a strong association with skin and soft tissue infections (33). A second risk factor for the SCCmec type IV phenotype was a shorter length of hospital stay before MRSA culture. This association may be due to the increased severity of illness and coexisting conditions in patients with a longer hospital stay, factors that have been commonly associated with the traditional (non-SCCmec type IV) HA-MRSA infections. However, measures of severity of illness and coexisting conditions were not captured in this investigation. A third risk factor was a later year of culture collection; the likelihood of SCCmec type IV phenotype peaked in 2003. The rise of these isolates in our hospital may be from CA-MRSA isolates brought in from colonized persons from the community. CA-MRSA infections in Los Angeles County have rapidly become common and now exceed the frequency of those caused by CA–methicillin-susceptible S. aureus (34). Alternatively, the rise of SCCmec type IV isolates may be a result of spread throughout our hospital by the usual means of dissemination in a healthcare setting (e.g., hands of healthcare workers, contaminated environment) (35) or possibly by a combination of factors.
Exactly why the SCCmec type IV strains are successful in hospital settings such as ours and others (20) is unknown. Some evidence indicates that SCCmec type IV strains may be more “fit” than SCCmec types II/III that contain HA-MRSA isolates. Compared with methicillin-susceptible S. aureus, isolates containing SCCmec type II/III replicate more slowly in vitro (36). Okuma et al. found that CA-MRSA isolates that contain SCCmec type IV replicate more rapidly than these traditional HA-MRSA strains and argued that CA-MRSA may have enhanced ecologic fitness compared with SCCmec type II/III isolates, perhaps due simply to a shorter doubling time (37). Given the vulnerable population within the hospital setting, it is unclear how infections with isolates that contain SCCmec type IV will differ in symptoms and severity from those caused by traditional HA-MRSA isolates. On the basis of our study and other somewhat similar reports (20), concern is rising that USA300 strains may overtake the traditional HA-MRSA strains in many hospital and healthcare settings.
Our investigation had some limitations. First, the analysis was retrospective and thus it was not possible to prospectively identify patients with HA infections and compare them with patients with CA infections. Although, by means of a chart review of a subset of patients who were selected by the criteria of a MRSA culture obtained ≥72 hrs after admission, none of these infections fulfilled the CDC definition of a CA-MRSA infection (31).
A second limitation was that our case definition was based on phenotypic criteria because nonbloodstream isolates had been discarded and the SCCmec type could not be validated. Traditionally, most HA-MRSA isolates in the United States carry SCCmec type II (and to a lesser extent, SCCmec type III) that encodes resistance to β-lactam antimicrobial agents bleomycin, macrolide-lincosamide-streptogamin B, aminoglycosides, and spectinomycin (38). Gentamicin resistance occurs in most strains that carry the SCCmec type II element but is conferred by the aac6′–aph2′′ gene elsewhere on the chromosome and is frequently carried by transposon Tn4001 (11,16). Therefore, to select for isolates that did not confer a phenotype typical of healthcare-associated or non-SCCmec type IV–containing isolates, the SCCmec type IV phenotype was defined as isolates that were resistant to oxacillin and susceptible to gentamicin, clindamycin, and trimethoprim-sulfamethoxazole.
Some banked isolates did not grow, and in 1 isolate we could not detect an SCCmec element. Of note, stored isolates may lose their SCCmec elements over time (39), which may explain our findings. Nevertheless, over the 6-year observation period of our investigation, among isolates, the phenotype and genotype definition of SCCmec type were in agreement for >90% of isolates. Thus, we were able to validate our case definition of an HA-MRSA isolate with SCCmec type IV phenotype using both chart review and SCCmec typing.
A third limitation of our investigation was that we were able to recover only bloodstream isolates, a subset of strains that are small and potentially nonrepresentattive. Whether the relationship of phenotype to genotype is similar for bloodstream and nonbloodstream infections is unclear. A fourth limitation is that all of the patients were from 1 institution and, therefore, may only reflect local trends. However, as previously mentioned, reports of isolates associated with the CA-MRSA infections causing HA infections are growing (17–20).
In summary, we found that over a 5-year span, MRSA with a CA-MRSA phenotype has become the most common cause of HA-MRSA infections in our institution. This finding has important implications for MRSA epidemiology, infection control practices, and empiric antimicrobial drug selection.
Dr Maree reports having received grant support from Pfizer Pharmaceuticals. Drs Daum and Boyle-Vavra have no conflicts of interest. Ms Matayoshi has no conflicts of interest. Dr Miller reports having received lecture and consulting fees from Pfizer Pharmaceuticals.
Dr Maree’s effort was supported by a grant from NIAID (T32 AI07481-09). Drs Daum and Boyle-Vavra’s efforts were supported by a grant from NIAID (AI40481-01A1), CDC (RO1 CCR523379), and the Grant Health Care Foundation. Dr Miller’s effort was supported by grants from CDC(RO1/CCR923419) and the National Institutes of Health (K23AI0183).
Dr Maree is a clinical and research fellow in Infectious Diseases at UCLA Medical Center. She is currently studying community-associated MRSA infections and completing her PhD in epidemiology at the University of California, Los Angeles, School of Public Health.
Acknowledgment
We are indebted to Danny Kim and Jie Peng for performing SCCmec typing, MLST typing, and PCR to detect the Panton-Valentine leukocidin genes. We thank Roger Detels for his continued support and guidance. In addition, we acknowledge Kevin Bui, Gunter Rieg, and Grace Tagudar for their important contributions to this investigation.
References
- Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance System (NNIS) report, data summary from January 1992 through June 2003, issued August 2003.Am J Infect Control. 2003;31:481–98. DOIPubMedGoogle Scholar
- Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey.Clin Infect Dis. 2005;40:562–73. DOIPubMedGoogle Scholar
- Chambers HF. Community-associated MRSA—resistance and virulence converge.N Engl J Med. 2005;352:1485–7. DOIPubMedGoogle Scholar
- Chambers HF. The changing epidemiology of Staphylococcus aureus?Emerg Infect Dis. 2001;7:178–82.PubMedGoogle Scholar
- Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002–2003.MMWR Morb Mortal Wkly Rep. 2003;52:88.
- Bancroft E, Kilgore G, Jones A, Yasuda L, Lee NE. < Ruane P, et al. Four outbreaks of community-associated methicillin-resistant Staphylococcus aureus in Los Angeles County. Presented at the 41st Annual Meeting of the Infectious Diseases Society of America; 2003 Oct 9–13; San Diego, California. Alexandria (VA): Infectious Diseases Society of America; 2003.
- Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers.Clin Infect Dis. 2004;39:971–9. DOIPubMedGoogle Scholar
- Eady EA, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections.Curr Opin Infect Dis. 2003;16:103–24.PubMedGoogle Scholar
- Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91.
- Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections.Emerg Infect Dis. 2005;11:928–30.PubMedGoogle Scholar
- Lelievre H, Lina G, Jones ME, Olive C, Forey F, Koussel-Delvallez M, Emergence and spread in French hospitals of methicillin-resistant Staphylococcus aureus with increasing susceptibility to gentamicin and other antibiotics.J Clin Microbiol. 1999;37:3452–7.PubMedGoogle Scholar
- Seal JB, Moreira B, Bethel CD, Daum RS. Antimicrobial resistance in Staphylococcus aureus at the University of Chicago Hospitals: a 15-year longitudinal assessment in a large university-based hospital.Infect Control Hosp Epidemiol. 2003;24:403–8. DOIPubMedGoogle Scholar
- Weber JT. Community-associated methicillin-resistant Staphylococcus aureus.Clin Infect Dis. 2005;41(Suppl 4):S269–72. DOIPubMedGoogle Scholar
- Denis O, Deplano A, Nonhoff C, De Ryck R, de Mendonca R, Roltiers S, National surveillance of methicillin-resistant Staphylococcus aureus in Belgian hospitals indicates rapid diversification of epidemic clones.Antimicrob Agents Chemother. 2004;48:3625–9. DOIPubMedGoogle Scholar
- Perez-Roth E, Lorenzo-Diaz F, Batista N, Moreno A, Mendez-Alvarez S. Tracking methicillin-resistant Staphylococcus aureus clones during a 5-year period (1998 to 2002) in a Spanish hospital.J Clin Microbiol. 2004;42:4649–56. DOIPubMedGoogle Scholar
- Donnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal cassette chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period.J Antimicrob Chemother. 2004;53:808–13. DOIPubMedGoogle Scholar
- Kourbatova EV, Halvosa JS, King MD, Ray SM, White N, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections.Am J Infect Control. 2005;33:385–91. DOIPubMedGoogle Scholar
- O’Brien FG, Pearman JW, Gracey M, Riley TV, Grubb WB. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak.J Clin Microbiol. 1999;37:2858–62.PubMedGoogle Scholar
- Saiman L, O’Keefe M, Graham PLIII, Wu F, Said-Salim B, Kreiswirth B, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women.Clin Infect Dis. 2003;37:1313–9. DOIPubMedGoogle Scholar
- Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections.Clin Infect Dis. 2006;42:647–56. DOIPubMedGoogle Scholar
- Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2001;45:1323–36. DOIPubMedGoogle Scholar
- Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk.JAMA. 1998;279:593–8. DOIPubMedGoogle Scholar
- Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates.Clin Infect Dis. 2003;37:1050–8. DOIPubMedGoogle Scholar
- Mongkolrattanothai K, Boyle S, Murphy TV, Daum RS. Novel non-mecA-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagF genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in Staphylococcus aureus.Antimicrob Agents Chemother. 2004;48:1823–36. DOIPubMedGoogle Scholar
- Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains.Antimicrob Agents Chemother. 2002;46:1147–52. DOIPubMedGoogle Scholar
- Suzuki E, Kuwahara-Arai K, Richardson JF, Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains.Antimicrob Agents Chemother. 1993;37:1219–26.PubMedGoogle Scholar
- Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV.J Clin Microbiol. 2005;43:4719–30. DOIPubMedGoogle Scholar
- Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC.Antimicrob Agents Chemother. 2004;48:2637–51. DOIPubMedGoogle Scholar
- Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus.J Clin Microbiol. 2000;38:1008–15.PubMedGoogle Scholar
- Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia.Clin Infect Dis. 1999;29:1128–32. DOIPubMedGoogle Scholar
- Minnesota Department of Health. Community-associated methicillin-resistant Staphylococcus aureus in Minnesota.Disease Control Newsletter.2004;32:61–72.
- Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant—Minnesota and North Dakota, 1997–1999.JAMA. 1999;282:1123–5. DOIPubMedGoogle Scholar
- Diep BA, Sensabaugh GF, Somboona NS, Carleton HA, Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin.J Clin Microbiol. 2004;42:2080–4. DOIPubMedGoogle Scholar
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, et al. Methicillin-resistant S. aureus infections among patients in the emergency department.N Engl J Med. 2006;355:666–74. DOIPubMedGoogle Scholar
- Mulligan ME, Murray-Leisure KA, Ribner BS, Standiford HC, John JF, Korvick JA, et al. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management.Am J Med. 1993;94:313–28. DOIPubMedGoogle Scholar
- Cribier B, Prevost G, Couppie P, Finck-Barbancon V, Grosshans E, Piemont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study.Dermatology. 1992;185:175–80.PubMedGoogle Scholar
- Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O’Brien FG, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community.J Clin Microbiol. 2002;40:4289–94. DOIPubMedGoogle Scholar
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of methicillin-resistant Staphylococcus aureus.Lancet. 2001;357:1225–40. DOIPubMedGoogle Scholar
- van Griethuysen A, van Loo I, van Belkum A, Vandenbroucke-Grauls C, Wannet W, van Keulen P, et al. Loss of the mecA gene during storage of methicillin-resistant Staphylococcus aureus strains.J Clin Microbiol. 2005;43:1361–5.DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1Findings from this investigation were presented in part at the 45th Annual International Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, December 2005.
2Current affiliation: University of Southern California School of Pharmacy, Los Angeles, California
Table of Contents – Volume 13, Number 2—February 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Loren G. Miller, Harbor-UCLA Medical Center, Division of Infectious Disease, 1000 W Carson St, Box 466, Torrance, CA 90509, USA: .
Top