Volume 13, Number 2—February 2007
Synopsis
Immune Cell Apoptosis Prevention as Potential Therapy for Severe Infections
Figure 1
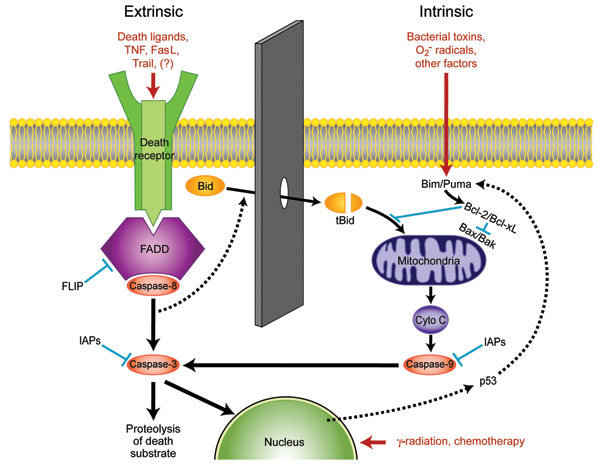
Figure 1. Apoptotic pathways of cell death. The extrinsic pathway is mediated by a variety of death receptor ligands, including tumor necrosis factor (TNF) and Fas ligand (FaSL), that trigger apoptosis by binding to cell surface receptors. In the intrinsic pathway, several adverse factors act upon mitochondria to cause loss of the mitochondrial membrane potential, resulting in leakage into the cytosol of cytochrome C (Cyto C), which together with apoptotic protease activating factor 1 forms the apoptosome that activates caspase-9. Communication between the pathways exists through cleavage of Bcl-2 interacting domain (Bid) by active caspase-8 to form truncated Bid (tBid). Inhibitors of apoptosis (IAPs) can prevent caspase activation under certain conditions. Trail, tumor necrosis factor-α–related apoptosis-inducing ligand; Bim/Puma, Bcl-2 interacting mediator of cell death/p53-upregulated modulator of apoptosis; FADD, Fas-associated death domain; FLIP, Fas-associated death domain-like interleukin-1β converting enzyme-like inhibitory protein.