Volume 13, Number 5—May 2007
Dispatch
Environmental Source of Candida dubliniensis
Cite This Article
Citation for Media
Abstract
We isolated Candida dubliniensis from a nonhuman source, namely, tick samples from an Irish seabird colony. The species was unambiguously identified by phenotypic and genotypic means. Analysis of the 5.8S rRNA gene showed that the environmental isolates belong to C. dubliniensis genotype 1.
The ever increasing number of immunosuppressed humans has led to a marked rise in opportunistic infections, particularly those caused by fungi (1). Candida albicans is the yeast species most commonly associated with oropharyngeal and systemic candidiasis in immunocompromised persons. However, the last 2 decades have seen an increase in infections by other Candida species, including C. dubliniensis, which was first recognized as distinct from C. albicans in 1995 in Ireland (2,3). C. dubliniensis has been recovered mainly from the oral cavities of HIV-infected persons (4) but also from lungs, vaginas, blood, and feces; occasionally this organism causes fatal systemic infections (5). Isolates are assigned to 4 genotypes, defined by the sequence of the internal transcribed spacer regions of the rRNA gene (6).
C. dubliniensis is globally distributed. In HIV-infected patients, the oral prevalence is 1.5%–32% (5). In healthy persons not infected with HIV, C. dubliniensis is absent or rare, but 14% of healthy Caucasians had oral C. dubliniensis in a South African study (7). Like C. albicans, C. dubliniensis may be a member of the normal oral microbial flora of humans, and oral candidosis may result from overgrowth of resident strains. In contrast to other Candida species, some of which are associated with birds (8,9), C. dubliniensis has not been found to date in nonhuman environmental sources. This has led to speculation that the species may be restricted to humans, possibly occupying sites deep within the oropharynx or upper respiratory tract (5).
Fungal strains were obtained from Ixodes uriae ticks (as part of a National Environment Research Council–funded study of a tickborne virus) at a seabird breeding colony on Great Saltee Island, Ireland (52°07′N, 6°36′W). The ticks were taken from cracks in cliffs used by common guillemots (Uria aalge). Tissue cultures of tick homogenates undertaken for virus isolation were occasionally contaminated with yeastlike fungi.
To investigate this, individual adult ticks were homogenized in 1 mL minimum essential medium (MEM). After centrifugation (30 s, 10,000× g), 0.2 mL of supernatant was added to 4 mL MEM, 5% fetal bovine serum, and 100 μg/mL penicillin-streptomycin. Cultures incubated at 37°C were examined microscopically daily for up to 6 days. Positive cultures were plated twice on Sabouraud dextrose agar (SAB) with chloramphenicol (BioMérieux, Marcy l’Etoile, France) before phenotypic testing. Isolates were identified by using API identification kits (BioMérieux) and by conventional methods (10). Antifungal drug susceptibility was tested according to the Clinical Laboratory Standards Institute guidelines (11). The control strains were C. albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, and C. dubliniensis NCPF 3949.
Internal transcribed spacer 1 and 2 regions (ITS1/ITS2) and the 5.8S rRNA gene were amplified with primers ITS1 and ITS4, described by White et al. (12). Template DNA was prepared by boiling single SAB-grown colonies in 50 μL ultrapure water for 10 min. After centrifugation (5 min 10,000× g), 15 μL supernatant was added to 50 μL PCRs containing 1× reaction buffer, 1 μmol/L ITS primers, 1.5 mmol/L MgCl2, 400 μmol/L deoxynucleoside triphosphates, and 2.5 U Immolase (BiolineLtd, London, England, UK). Cycling parameters were 7 min 95°C, 30 cycles 1 min 95°C, 1 min 58°C, 1 min 72°C, and a final extension of 5 min 72°C. Products were purified (QiaQuick kit, QIAGEN Ltd., West Sussex, England, UK) and sequenced (BigDye kit and ABI 377 sequencer; Applied Biosystems, Foster City, CA, USA) by using the ITS primers. Sequences were assembled by using Lasergene 6, Seqman version II, and aligned by using BioEdit software (13).
Fungal isolation was undertaken on 2 separate days with samples from 2 distinct sites on the island (Table 1). On both days, Happy Hole West (HHW) ticks were processed immediately after Labour in Vain (LIV) ticks in the same class II microbiologic cabinet. No fungi were detected in HHW ticks, whereas 16.7%–27.6% of ticks sampled from 2 locations within LIV gave positive cultures (Table 1). Twenty-two isolates were obtained (Table 1); SL370–429 were from LIV-1 and SL495–531 from LIV-2 (SL = Saltee).
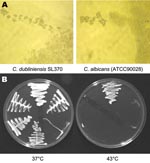
On SAB the colonies from positive cultures were a creamy white color with a glabrous appearance similar to C. albicans. SL375 had a mixed phenotype (large and small colonies, designated SL375–1 and SL375–2). Like C. albicans, all SL isolates were germ-tube positive and produced chlamydospores at 37°C on Corn Meal Tween 80 agar (Oxoid Ltd, Basingstoke, England, UK) and Czapek Dox (1%) Tween 80 agar (Oxoid) (Figure 1A). None of the SL isolates grew at 43°C on SAB (Figure 1B), which suggests that they might be C. dubliniensis (14). This was confirmed by carbohydrate assimilation tests (Table 2) and by sequencing the 5.8S rRNA gene (Figure 2). With the API 20C AUX kit, all SL isolates yielded the same profile at 48 h, interpreted as 99.9% C. dubliniensis (Table 2). Eleven isolates from LIV-1 and the C. dubliniensis (NCPF 3949) reference strain were also tested with API 32C. All had an identical profile (7143100015), interpreted as 81.9% C. dubliniensis and 16.9% C. albicans.
ITS sequences of isolates SL375, SL397, SL407, SL410, SL411, SL417, and SL422 were identical to that of C. dubliniensis CD33 genotype 1 (Figure 2). Nevertheless, phenotypic variation among the SL isolate was evident. In addition to variation in trehalose assimilation rates, 3–4 distinct types were apparent in the germ tube test. Three independent inoculations (105 CFU/mL, mid log growth phase) of each isolate gave consistent morphologic differences. One group produced very long germ tubes (SL375–1, SL422, SL417 SL387, SL397, and SL413); another, medium size (SL411, SL407); a third, mainly long germ tubes with cells that aggregate like C. parapsilosis (SL375–2 and SL410); and the last, elongated cigarlike cells that clamped together with few germ tubes (SL370 and SL371).
To determine whether C. dubliniensis was present on their outer surface LIV-2 and HHW-2 ticks (n = 102) were individually washed in 1 mL MEM before homogenization. C. dubliniensis was cultured from the wash supernatants of 8 of 9 ticks that proved positive after homogenization but not from any of the negative ticks. These findings strongly suggest that the fungus is present in particles adhering to the ticks.
The SL isolates (n = 11) were extremely sensitive to antifungals. MIC90s (μg/mL) were as follows: amphotericin B 0.031 (Sigma-Aldrich, Saint Louis, MO, USA), flucytosine 0.031 (Valeant Pharmaceuticals International, Aliso Viejo, CA, USA), fluconazole 0.125 (Pfizer Inc., Sandwhich, Kent, England, UK), itraconazole 0.007 (Ortho Biotech, Bridgewater, NJ, USA), voriconazole 0.007 (Pfizer), posaconazole 0.007 (Schering-Plough, Kenilworth, NJ, USA), ketoconazole <0.007 (Ortho Biotech), and caspofungin <0.007 (Merck & Co., Inc., Rahway, NJ, USA).
Our serendipitous isolation of C. dubliniensis from the environment ends speculation (5) that the species might be confined to humans. Because ticks were collected from cracks filled with guillemot guano and the fungal isolates were associated with the surface of ticks, the most likely source of the fungus is bird excrement. Thus, like C. albicans (8), C. dubliniensis may inhabit the digestive tract of marine birds. Only C. dubliniensis was isolated from the Great Saltee ticks; however, we have detected other Candida spp. from both soil and tick samples (unpub. data) at other seabird colonies, which suggests that marine as well as terrestrial birds (9) carry a variety of yeast species (15).
The environmental isolates are members of human C. dubliniensis genotype 1, commonly associated with HIV patients (6). Sequencing of the genotype 1 CD36 isolated from an HIV patient is nearly complete (www.sanger.ac.uk/sequencing/Candida/dubliniensis). Phylogenetic comparison of variable loci from environmental and human genotype 1 isolates could be used to estimate when they last shared a common ancestor and thus how often C. dubliniensis is transmitted from the environment to humans.
Dr Nunn heads the Tick and Tick-Borne Pathogen Research Group at the Centre for Ecology and Hydrology in Oxford. His research interests focus on the evolution and transmission of tickborne pathogens and functional characterization of tick immunomodulators.
Acknowledgments
We thank Stuart Murray for collecting tick and soil samples from Great Saltee Island and Guido Paesen for reviewing the manuscript.
This work was supported by the National Environment Research Council Environmental Genomics and Biodiversity Programs.
References
- Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–31. DOIPubMedGoogle Scholar
- Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DA, Coleman DC. Candida dubliniensis sp. nov.:phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–21. DOIPubMedGoogle Scholar
- Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–34.PubMedGoogle Scholar
- Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Llloyd S, Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus–infected individuals. J Clin Microbiol. 1997;35:960–4.PubMedGoogle Scholar
- Sullivan DJ, Moran GP, Coleman DC. Candida dubliniensis: ten years on. FEMS Microbiol Lett. 2005;253:9–17.PubMedGoogle Scholar
- Gee SF, Joly S, Soll DR, Meis JFGM, Verweij PE, Polacheck I, Identification of four distinct genotypes of Candida dubliniensis and detection of microevolution in vitro and in vivo. J Clin Microbiol. 2002;40:556–74. DOIPubMedGoogle Scholar
- Blignaut E, Pujol C, Joly S, Soll DR. Racial distribution of Candida dubliniensis among South Africans. J Clin Microbiol. 2003;41:1838–42. DOIPubMedGoogle Scholar
- Buck JD. Isolation of Candida albicans and halophilic Vibrio spp. from aquatic birds in Conneticut and Florida. Appl Environ Microbiol. 1990;56:826–8.PubMedGoogle Scholar
- Cafarchia C, Camarda A, Romito D, Campolo M, Quaglia NC, Tullio D, Occurrence of yeast in cloacae of migratory birds. Mycopathologia. 2006;161:229–34. DOIPubMedGoogle Scholar
- Warren NG, Hazen KC. Candida, Cryptococcus and other yeasts of medical importance. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Volken RH, editors. Manual of clinical microbiology, 7th ed. Washington: ASM Press; 1999. p. 1184–99.
- NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeast, approved standard M27-A. Wayne (PA): National Committee for Clinical Laboratory Standards; 1997.
- White TJ, Bruns TD, Lee SB, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sininsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press Inc.; 1990. p. 315–22.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8.
- Gales AC, Pfaller MA, Houston AK, Joly S, Sullivan DJ, Coleman DC, Identification of Candida dubliniensis based on temperature and utilization of xylose and alpha-methyl-D-glucosidase as determined by API 20C AUX and Vitek YBC systems. J Clin Microbiol. 1999;37:3804–8.PubMedGoogle Scholar
- Buck JD. A note on the experimental uptake and clearance of Candida albicans in a young captive gull (Larus sp.). Mycopathologia. 1986;94:59–61. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 5—May 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Miles A. Nunn, National Environment Research Council Centre for Ecology and Hydrology, Mansfield Rd, Oxford OX1 3SR, England, United Kingdom;
Top