Volume 13, Number 9—September 2007
Dispatch
Sympatric Occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand
Cite This Article
Citation for Media
Abstract
We confirmed sympatric occurrence of Taenia solium, T. saginata, and T. asiatica in western Thailand. DNA analysis of morphologically identified T. saginata, in a dual infection with T. solium, indicated it was T. asiatica. To our knowledge, this report is the first of T. asiatica and a dual Taenia infection from Thailand.
Taeniid tapeworm infections in the human intestine are caused by Taenia solium, T. saginata, and T. asiatica in Asia and the Pacific (1–3). Taeniasis caused by T. solium is a serious public health problem worldwide because eggs and proglottids expelled in the stool can infect humans through contamination of the environment and cause fatal neurocysticercosis. Neurocysticercosis cases caused by T. solium have increased in non–taeniasis-endemic areas (3–5).
A related taeniid tapeworm, Asian Taenia (= T. asiatica), has been described in Taiwan and the Republic of Korea (1–3,6–8). Although T. asiatica is phylogenetically closely related and is considered to be a sister species of T. saginata (1–3,6,7), the important intermediate host for T. asiatica is the domestic pig and the metacestodes mainly develop in the pigs’ liver (6). The morphologic characteristics of adult T. asiatica are very similar to those of T. saginata. Morphologic differentiation by either scolex or gravid proglottid of these 2 species is practically impossible (1,3). On the basis of molecular analysis of taeniid isolates from Asia and the Pacific, T. asiatica is distributed in Taiwan, the Republic of Korea, Malaysia, People’s Republic of China, Philippines, Indonesia, and Vietnam (1–3,6–11). However, there has been no evidence of the distribution of T. asiatica in Thailand (8,12).
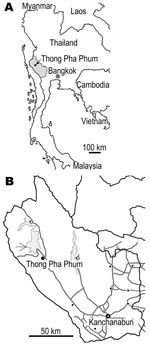
The field investigation was conducted during 2002–2005 in communities in Thong Pha Phum District, 150 km northwest of Kanchanaburi Province, Thailand, close to the Myanmar border (Figure 1). The region is mountainous terrain that acts as a natural border between Thailand and Myanmar, and it mostly comprises natural parks and a water reservoir. The major studied population is Karen; Mon and Thai are minorities. In our study, molecular identification of these taeniid samples was conducted in Asahikawa, Japan in 2006. The study team, the Faculty of Tropical Medicine at Mahidol University, obtained ethical approval for a human stool survey for control of helminthic infections.
All taeniasis patients were informed of the study objectives and worm collection procedure through discussions, including about expulsion of proglottids. Tapeworms, either with scolices or without scolices, were expelled from 24 persons with taeniasis after the persons received 2 g of niclosamide or 40 mg/kg of praziquantel and a purgative. A total of 29 scolices were confirmed. Scolices with hooks expelled from 5 patients (nos. 2, 4, 7, 9, 11) were identified as T. solium. Scolices without hooks from 12 patients were identified as T. saginata because no molecular evidence on the distribution of T. asiatica in Thailand exists and all Taenia scolices without hooks were confirmed to be T. saginata in other areas in Thailand (8,10,12). Morphologic identification of the species was based on scolex only. Specimens without scolices were not identified morphologically. Most patients (12/17) harbored a single scolex. However, several patients harbored 2–6 scolices, including 1 dual infection with 2 T. solium and 1 T. saginata (patient 7) (Table). Patients were 7–60 years of age; 16 were male, and 8 were female.
Nineteen Taenia samples were fixed in 80% ethanol and kept at –20°C until use. Four samples were fixed in 10% formalin. All scolices were fixed with alcohol-formalin-acetic acid and stained with acetocarmine for morphologic comparative examination. One scolex with or without hooklets each from a patient 7 was further processed for molecular studies.
DNA samples were extracted from taeniid proglottids except for patient 7, for whom DNA was extracted from 2 scolices. DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) was used for the samples kept in ethanol. A DNA Isolator PS kit (Wako Pure Chemicals, Osaka, Japan) and DEXPAT (TaKaRa Shuzo, Shiga, Japan) were used for the formalin-fixed proglottids. DNA samples from 2 scolices expelled from patient 7 stained with acetocarmine were prepared by using DEXPAT and 0.05 N NaOH/1% sodium dodecyl sulfate containing proteinase K. Mitochondrial DNA diagnosis of ethanol-fixed samples was performed by multiplex PCR by using cytochrome c oxidase subunit 1 gene (cox1), except for the use of a forward primer (5′-TTATTTATTTACGTCAATCTTATTG-3′, positions 561–585) for T. asiatica (10). The formalin-fixed and acetocarmine-stained specimens were identified by base excision sequence scanning thymine-base (BESS T-base) analysis that used either cox1 or cytochrome b gene (cob) (13). For BESS T-base analysis, the following primers were used: F3 (5′-TATTTGATCGTAAATTTAGTTCT-3′, corresponding to nucleotide (nt) positions 629–651) and R7 (5′-ATTAACACATAAACCTCGGGA-3′, nt positions 740–720) for cox1 of T. solium, F1 (5′-GTCAAAAGATTCTTTTTTTACTTGGT-3′, nt positions 180–205) and R2 (5′-CCCTTCTTTCTATAACTTGAATAAT-3′, nt positions 305–281) for cob for T. solium. DNA sequencing of the products amplified by multiplex PCR was performed for confirmation. DNA samples for sequencing were prepared with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). DNA sequencing was performed on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems), and the nucleotide sequence data were analyzed by using DNASTAR version 3.75 (DNASTAR Inc., Madison, WI, USA).
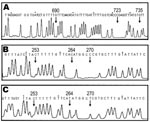
Multiplex PCR applied on 19 proglottids from 17 patients with cox1 (10) showed that 5, 7, and 7 proglottids were T. solium (Asian genotype) (10,13), T. saginata, and T. asiatica, respectively (data not shown). These results were supported by DNA sequencing of the amplicons (data not shown). By contrast, small sizes of 112-bp cox1 products were successfully amplified from samples taken from patients 3–6. These samples had been preserved in 10% formalin for years and BESS T-base analysis indicated that they were T. solium (Asian genotype) (Figure 2A) (14). BESS T-base analysis showed that scolices with and without hooklets from a dual infection (patient 7) were T. solium (Asian genotype) and T. asiatica, respectively (Figure 2B and C). To our knowledge, this is the first report demonstrating a dual infection with T. solium and T. asiatica in which 3 human taeniid cestodes are sympatrically distributed (1).
We documented sympatric distribution of T. solium, T. saginata, and T. asiatica in western Thailand on the basis of mitochondrial DNA analysis. Our study indicated that 53.3% (8/15) of taeniid specimens expected to be T. saginata were T. asiatica and that both T. asiatica and T. saginata are codistributed in Kanchanaburi Province. Although T. solium taeniasis has seldom been reported in the literature in Thailand (15), our study has shown infection with T. solium (Asian genotype) in 11 (45.8%) of 24 Taenia-infected patients. The number of T. solium organisms expelled from taeniasis patients varied from 1 to 6, and >2 tapeworms were found in 36.4% (4/11) of T. solium taeniasis patients. In addition, we confirmed a dual infection with T. solium and T. asiatica (in patient 7). This experience indicates that molecular analysis is preferable and necessary for precise re-identification of so-called T. saginata in Asia and the Pacific (1).
Although T. solium cysticercosis in humans has not been reported in this study area, these populations appear to pose a risk for environmental contamination and person-to-person spread of T. solium leading to cysticercosis in humans and swine. Raw or inadequately cooked beef, pork, or pig viscera, and fresh blood are commonly consumed by local people in the study areas, and consequently they are at high risk of acquiring taeniasis. Therefore, to improve sanitation and quality of life, sustainable health education should be introduced and stressed to the population in the community.
Dr Anantaphruti is an associate professor in the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Her research interests include epidemiology, drug trials, and immunodiagnosis of helminthic infections, particularly cestode zoonoses.
Acknowledgments
We thank Vajiralongkorn Dam for accommodations during our field work and Peter M. Schantz for his comments and suggestions for improving this article.
The field survey in Thailand from 2002 until 2005 was funded by Mahidol University research grant 02011285-0002 to J.W. The molecular work was supported by a grant-in-aid for Scientific Research from the Japan Society for Promotion of Science (JSPS) to A.I. (14256001, 17256002) and to M.O. (18406008) and by a JSPS-Asia/Africa Sciences Platform Fund (2006–2008) to A.I.
References
- Ito A, Nakao M, Wandra T. Human taeniasis and cysticercosis in Asia. Lancet. 2003;362:1918–20. DOIPubMedGoogle Scholar
- Ito A, Wandra T, Yamasaki H, Nakao M, Sako Y, Nakaya K, Review: cysticercosis/taeniasis in Asia and the Pacific. Vector Borne Zoonotic Dis. 2004;4:95–107. DOIPubMedGoogle Scholar
- Ito A, Craig PS, Schantz PM. Taeniasis/cysticercosis and echinococcosis with focus on Asia and the Pacific. Parasitol Int. 2006;55:S1–308. DOIPubMedGoogle Scholar
- Schantz PM, Moore AC, Munoz JL, Hartman BJ, Schaefer JA, Aron AM, Neurocysticercosis in an orthodox Jewish community in New York City. N Engl J Med. 1992;327:692–5.PubMedGoogle Scholar
- Hira PR, Francis I, Abdella NA, Gupta R, Ai-Ali FM, Grover S, Cysticercosis: imported and autochthonous infections in Kuwait. Trans R Soc Trop Med Hyg. 2004;98:233–9. DOIPubMedGoogle Scholar
- Eom KS, Rim HJ. Morphological descriptions of Taenia asiatica sp. n. Korean J Parasitol. 1993;31:1–6. DOIPubMedGoogle Scholar
- Bowles J, McManus DP. Genetic characterization of the Asian Taenia, a newly described taeniid cestode of humans. Am J Trop Med Hyg. 1994;50:33–44.PubMedGoogle Scholar
- De NV, Hoa LT, Doanh NQ, Ngoc NB, Cong LD. Report on a new species of Taenia (Taenia asiatica) in Hanoi, Vietnam. J Malaria Parasit Dis Control. 2001;3:80–5.
- Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol. 2004;42:548–53. DOIPubMedGoogle Scholar
- Wandra T, Depary AA, Sutisna P, Margono SS, Suroso T, Okamoto M, Taeniasis and cysticercosis in Bali and North Sumatra, Indonesia. Parasitol Int. 2006;55:S155–60. DOIPubMedGoogle Scholar
- Morakote N, Wijit A, Uparanukraw P. Further search for Taenia saginata asiatica in Chiang Mai, Thailand. Ann Trop Med Parasitol. 2000;94:521–4.PubMedGoogle Scholar
- Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology. 2002;124:657–62. DOIPubMedGoogle Scholar
- Yamasaki H, Nakao M, Sako Y, Nakaya K, Sato MO, Mamuti W, DNA differential diagnosis of human taeniid cestodes by base excision sequence scanning thymine-base reader analysis with mitochondrial genes. J Clin Microbiol. 2002;40:3818–21. DOIPubMedGoogle Scholar
- Anantaphruti MT. Human taeniasis in Thailand. In: Ito A, Wen H, Yamasaki H, editors. Asian Parasitology, vol. 2. Taeniasis/cysticercosis and echinococcosis in Asia, Chiba (Japan): FAP Journal Ltd; 2005. p. 89–98.
Figures
Table
Cite This ArticleTable of Contents – Volume 13, Number 9—September 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Akira Ito, Department of Parasitology, Asahikawa Medical College, Midorigaoka-Higashi 2-1-1-1, Asahikawa 078-8510, Japan;
Top