Volume 14, Number 1—January 2008
Dispatch
Protochlamydia naegleriophila as Etiologic Agent of Pneumonia
Abstract
Using ameba coculture, we grew a Naegleria endosymbiont. Phenotypic, genetic, and phylogenetic analyses supported its affiliation as Protochlamydia naegleriophila sp. nov. We then developed a specific diagnostic PCR for Protochlamydia spp. When applied to bronchoalveolar lavages, results of this PCR were positive for 1 patient with pneumonia. Further studies are needed to assess the role of Protochlamydia spp. in pneumonia.
Recently, a Naegleria endosymbiont (KNic) was observed but remained uncultivable, precluding precise identification (1). We grew a large amount of strain KNic by using Acanthamoeba castellanii, which enabled phenotypic, genetic and phylogenetic analyses that supported its affiliation as Protochlamydia naegleriophila. This new ameba-resistant intracellular bacteria might represent a new etiologic agent of pneumonia because it is likely also resistant to human alveolar macrophages (2,3). Because other Parachlamydiaceae were associated with lung infection (4–6), we assessed the role of Pr. naegleriophila in pneumonia by developing a diagnostic PCR and applying it to bronchoalveolar lavages.
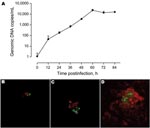
KNic growth in A. castellanii was assessed by immunofluorescence with in-house mouse anti-KNic and Alexa488-coupled anti-immunoglobulin antibodies (Invitrogen, Eugene, OR, USA). Confocal microscopy (LSM510; Zeiss, Feldbach, Switzerland) confirmed the intracellular location of KNic and demonstrated its rapid growth within A. castellanii. To precisely assess the growth rate, we performed PCR on A. castellanii/KNic coculture by using PrF/PrR primers and PrS probe. After 60 hours, we observed an increased number of bacteria per microliter of 4 logarithms (Appendix Figure).
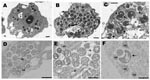
A. castellanii/KNic and N. lovaniensis/KNic cocultures were processed for electron microscopy as described. Ameba filled with bacteria exhibiting 3 developmental stages already described in other Parachlamydiaceae (8) were observed (Figure 1).
To measure the serologic differentiation index (SDI) between strain KNic and other Chlamydia-like organisms, we immunized Balb/c mice to produce anti-KNic antibodies. Purified Pr. amoebophila (ATCC PRA-7), Simkania negevensis (ATCC VR-1471), Parachlamydia acanthamoebae strain Seine, Waddlia chondrophila (ATCC 1470), Neochlamydia hartmannellae (ATCC 50802), Criblamydia sequanensis (CRIB 18), and Rhabdochlamydia crassificans (CRIB 01) antigens were tested by micro-immunofluorescence against mouse anti-KNic antibodies, whereas KNic antigen was tested with serum against all these different Chlamydia-like organisms. SDIs were calculated as described. Serum from mice immunized with KNic showed strong reactivity against autologous antigen (titers of 4,096). Significant cross-reactivity between KNic and Pr. amoebophila (SDI = 7) and P. acanthamoebae (SDI = 10) was observed. Mouse anti-KNic serum did not react with other Chlamydia-like organisms (Table 1). Because cross-reactivity between members of the order Chlamydiales was proportional to the relatedness between each species, the strong cross-reactivity between KNic and Pr. amoebophila supports the affiliation of KNic in the genus Protochlamydia.
Taxonomic position of KNic was further defined by sequencing 16Sr RNA (rrs, DQ635609) and ADP/ATP translocase (nnt, EU056171) encoding genes. The rrs was amplified/sequenced using 16SIGF/RP2Chlam primers. The nnt was amplified/sequenced using nntF2p (5′-TGT(AT)GAT(CG)CATGGCAA(AG)TTTC-3′) and nntR1p (5′-GATTT(AG)CTCAT(AG)AT(AG)TTTTG-3′) primers. Genetic and phylogenetic analyses were conducted by using MEGA software (12). The 1,467-bp rrs sequence showed 97.6% similarity with Pr. amoebophila, 91.8%–93.2% with other Parachlamydiaceae, and 85.7%–88.6% with other Chlamydiales. Based on the Everett genetic criteria (13), KNic corresponds to a new species within the Protochlamydia genus because its sequence similarity with Pr. amoebophila is >95% (same genus) and <98.5% (different species). Phylogenetic analyses of rrs gene sequences showed that KNic clustered with Pr. amoebophila, with bootstraps of 98% and 95% in neighbor-joining and minimum-evolution trees, respectively. The 569-bp nnt sequence exhibited 91.1% similarity with Pr. amoebophila, 65.5%–72.6% with other Parachlamydiaceae, and 55.4%–72.6% with other Chlamydiales. Phylogenetic analyses of nnt sequences showed that KNic clustered with Pr. amoebophila. On the basis of these analyses, we propose to name strain KNic “Protochlamydia naegleriophila.”
We then developed a specific diagnostic PCR for Protochlamydia spp. Primers PrF (5′-CGGTAATACGGAGGGTGCAAG-3′) and PrR (5′-TGTTCCGAGGTTGAGCCTC-3′) as well as probe PrS (5′-TCTGACTGACACCCCCGCCTACG-3′) were selected. The 5′-Yakima-Yellow probe (Eurogentec, Seraing, Belgium) contained locked nucleic acids (underlined in sequence above). The reactions were performed with 0.2 μM each primer, 0.1 μM probe, and iTaqSupermix (Bio-Rad, Rheinach, Switzerland). Cycling conditions were as described, and PCR products were detected with ABIPrism7000 (Applied Biosystems, Rotkreuz, Switzerland). Each sample was amplified in duplicate. Inhibition, negative PCR mixture, and extraction controls were systematically tested.
To allow quantification, a plasmid containing the target gene was constructed by cloning PCR products into pCR2.1-TOPO vector (Invitrogen, Basel, Switzerland). Recombinant plasmid DNA quantified using Nanodrop ND-1000 (Witech, Littau, Switzerland) was 10-fold diluted and used as positive controls.
The analytical sensitivity was 10 copy/μL (Figure 2, panel A). Intra-run variability was good (Figure 2 panel B) with a Bland-Altman bias of 0.99 and a limit of agreement of 2.87 (Figure 2, panel A). Inter-run variability was low at high concentration, 1.12, 1.71, 0.82, 1.77 cycles for 105, 104, 103, 102 copies/μL, respectively. Inter-run variability was higher at low concentration, 4.22 cycles for 101 copies/μL (Figure 2, panel A). Analytical specificity was tested with bacterial and eukaryotic DNA (Table 2). The PCR slightly amplified DNA from R. crassificans, another Chlamydia-like organism. No cross-amplification was observed with any other bacteria or with human cells. The absence of cross-amplification of P. acanthamoebae is important because this Chlamydia-related bacteria is considered an emerging agent of pneumonia (4–6).
We tested 134 bronchoalveolar lavage samples from patients with (n = 65) and without (n = 69) pneumonia and extracted DNA by using a Bio-Rad Tissue Kit. One sample was positive, with 543 and 480 copies/μL. This positive result was confirmed using the 16sigF/16sigR PCR (13), which targets another DNA segment. This sequence exhibited 99.6% (284/285) similarity with Pr. naegleriophila strain KNic and 95.1% (269/283) with Pr. amoebophila. The presence of Protochlamydia antigen in the sample was confirmed by immunofluorescence performed using rabbit anti-KNic antibody directly on the bronchoalveolar lavage sample and by ameba coculture (Appendix Figure).
The positive sample was taken from an immunocompromised patient who had cough, dyspnea, and a lung infiltrate. Bronchoscopy examination of the lower respiratory tract showed mucosal inflammation localized at the middle lung lobe. Cytology and Gram stain of the bronchoalveolar lavage showed many leucocytes with macrophages (65%) and neutrophils (23%). Although no antimicrobial treatment was administered prior to bronchoscopy, no other etiologic agent was identified despite extensive microbiologic investigations of bronchial aspirate and bronchoalveolar lavage. Results of Gram stain, auramine stain (for Mycobacterium spp.), and silver stain (for Pneumocystis carinii) tests were negative. Only physiologic oropharyngeal flora could be grown on sheep-blood and chocolate-bacitracin agars. Cell culture, as well as culture for fungi and mycobacteria, remained sterile. Moreover, results of PCRs specific for the detection of Legionella pneumophila, Chlamydophila pneumoniae, and Mycoplasma pneumoniae (15) were all negative. The patient recovered and remained free of symptoms of acute lung infection during the next 20 months.
Isolating new species from environmental and clinical samples is important to better define their epidemiology and potential pathogenicity. We defined the taxonomic position of a novel Naegleria endosymbiont and proposed its affiliation within the Protochlamydia genus as Pr. naegleriophila sp. nov. Moreover, we developed a new PCR targeting Protochlamydia spp., applied it to clinical samples, and identified a possible role of Pr. naegleriophila as an agent of pneumonia.
Protochlamydia naegleriophila (nae.gle.rio′.phi.la Gr. fem.n. Naegleria, name of host cell, Gr. adj. philos, -a friendly to, referring to intracellular growth of Protochlamydia naegleriophila strain KNic within Naegleria amebae). The 16Sr RNA sequence (DQ635609) of KNic is 97.6% similar to that of P. amoebophila, making this organism a member of the genus Protochlamydia. KNic does not grow on axenic media (1) but grows by 4 logarithms in 60 h within A. castellanii. KNic exhibits a Chlamydia-like developmental cycle, with reticulate, elementary, and crescent bodies. The reticulate body is about 900 nm and has a spiny appearance similar to that of P. amoebophila (Figure 2, panel B). To be classified within the Pr. naegleriophila species, a new strain should show a 16Sr RNA similarity >98.5% (13) and similar phenotypic traits.
Ms Casson is completing a PhD thesis at the University of Lausanne. Her research is dedicated to defining the role of the obligate intracellular Chlamydia-like organisms to humans, discovering new species, and defining their role in pneumonia.
Acknowledgments
We thank J.L. Barblan and the staff of Pôle Facultaire Médical Universitaire at the Medical Faculty of Geneva for assisting with electron microscopy analysis, staff of the Cellular Imaging Facility for assisting with confocal microscopy analysis, Gerhild Gmeiner for technical assistance in preparing Naegleria lovaniensis with Protochlamydia strain KNic for electron microscopy, Philip Tarr for reviewing the manuscript, and M. Perrenoud and S. Aeby for technical help.
This work was supported by the Swiss National Science Foundation grants FN 3200BO-105885 and FN 3200BO-116445. G.G. is supported by the Leenards Foundation through a career award entitled “Bourse Leenards pour la relève académique en médecine clinique à Lausanne.”
References
- Michel R, Muller KD, Hauröder B, Zöller L. A coccoid bacterial parasite of Naegleria sp. (Schizopyrenida: Vahlkampfiidae) inhibits cyst formation of its host but not transformation to the flagellate stage. Acta Protozool. 2000;39:199–207.
- Greub G, Mege JL, Raoult D. Parachlamydia acanthamebae enters and multiplies within human macrophages and induces their apoptosis. Infect Immun. 2003;71:5979–85. DOIPubMedGoogle Scholar
- Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–33. DOIPubMedGoogle Scholar
- Greub G, Raoult D. Parachlamydiaceae: potential emerging pathogens. Emerg Infect Dis. 2002;8:625–30.PubMedGoogle Scholar
- Greub G, Berger P, Papazian L, Raoult D. Parachlamydiaceae as rare agents of pneumonia. Emerg Infect Dis. 2003;9:755–6.PubMedGoogle Scholar
- Greub G, Boyadjiev I, La Scola B, Raoult D, Martin C. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann N Y Acad Sci. 2003;990:311–9.PubMedGoogle Scholar
- Casson N, Medico N, Bille J, Greub G. Parachlamydia acanthamoebae enters and multiplies within pneumocytes and lung fibroblasts. Microbes Infect. 2006;8:1294–300. DOIPubMedGoogle Scholar
- Greub G, Raoult D. Crescent bodies of Parachlamydia acanthamoebae and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl Environ Microbiol. 2002;68:3076–84. DOIPubMedGoogle Scholar
- Casson N, Entenza JM, Greub G. Serological cross-reactivity between different Chlamydia-like organisms. J Clin Microbiol. 2007;45:234–6. DOIPubMedGoogle Scholar
- Fang R, Raoult D. Antigenic classification of Rickettsia felis by using monoclonal and polyclonal antibodies. Clin Diagn Lab Immunol. 2003;10:221–8. DOIPubMedGoogle Scholar
- Thomas V, Casson N, Greub G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoeba coculture. Environ Microbiol. 2006;8:2125–35. DOIPubMedGoogle Scholar
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–63. DOIPubMedGoogle Scholar
- Everett KD, Bush RM, Andersen AA. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49:415–40.PubMedGoogle Scholar
- Jaton K, Bille J, Greub G. A novel real-time PCR to detect Chlamydia trachomatis in first-void urine or genital swabs. J Med Microbiol. 2006;55:1667–74. DOIPubMedGoogle Scholar
- Welti M, Jaton K, Altwegg M, Sahli R, Wenger A, Bille J. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae in respiratory tract secretions. Diagn Microbiol Infect Dis. 2003;45:85–95. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 14, Number 1—January 2008
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Gilbert Greub, Center for Research on Intracellular Bacteria, Institute of Microbiology, University Hospital Center and University of Lausanne, 1011 Lausanne, Switzerland;
Top