Volume 14, Number 7—July 2008
CME ACTIVITY - Research
Alcaligenes xylosoxidans Bloodstream Infections in Outpatient Oncology Office
Cite This Article
Citation for Media
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 0.5 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.com/cme/eid; (4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants will be able to:
- Identify properties of Alcaligenes xylosoxidans.
- Describe the clinical presentation of A. xylosoxidans infection in the current study.
- Specify risk factors for infection with A. xylosoxidans.
- Identify the primary source of infection with A. xylosoxidans in the current study.
Editor
D. Peter Drotman, MD, Editor-in-Chief, Emerging Infectious Diseases. Disclosure: D. Peter Drotman, MD, has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Associate Professor; Residency Director, Department of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed that he has served as an advisor or consultant to Novartis, Inc.
Authors
Disclosures: Moon Kim, MD, MPH; Elizabeth Bancroft, MD, SM; Eleanor Lehnkering, MS; and Rodney M. Donlan, PhD, have disclosed no relevant financial relationships. Laurene Mascola, MD, MPH, has disclosed that she has served has an advisor or consultant to Merck and MedImmune. Dr. Mascola has also disclosed that she has served as a speaker for Merck.
Abstract
In 2002, we investigated a cluster of patients with Alcaligenes xylosoxidans bloodstream infections by conducting a matched case–control study and a prospective study. Pulsed-field gel electrophoresis (PFGE) was performed on blood culture isolates, and 1 explanted central venous catheter (CVC) was tested for biofilm. We identified 12 cases of A. xylosoxidans bloodstream infection. Case-patients were more likely than controls to have had a CVC (7/7 [100%] vs 4/47 [8.7%], respectively; p<0.0001). Ten case isolates were indistinguishable by PFGE analysis, and A. xylosoxidans biofilm from the CVC matched the outbreak strain. We observed multiple breaches in infection control, which may have caused contamination of multidose vials used to flush the CVCs. Our study links A. xylosoxidans with CVC biofilm and highlights areas for regulation and oversight in outpatient settings.
Alcaligenes xylosoxidans, also known as Achromobacter xylosoxidans, is a gram-negative, water-borne organism that causes healthcare-associated infections (1–7) and bacteremia in immunocompromised patients with indwelling catheters (6–11); it can also contaminate liquids (2,5,12–14). A. xylosoxidans is found in soil and water and grows in saline (15,16). On January 16, 2002, the Acute Communicable Disease Control (ACDC) program of the Los Angeles County Department of Public Health was notified by a local hospital epidemiologist about a cluster of patients with A. xylosoxidans bloodstream infections (17). The patients had been admitted to Hospital A over a period of 2 months.
To confirm the presence of an outbreak, ACDC conducted a telephone survey, which asked the microbiology laboratories of Hospital A and 4 surrounding hospitals for a list of all patients who had had positive blood cultures for A. xylosoxidans in the past 3 months. One laboratory identified 3 such patients, and Hospital A laboratory identified 7; all 9 patients (1 case-patient had positive blood cultures reported from both laboratories) were associated with a single outpatient oncology office, Office B. The other 3 hospitals reported that they had not identified any A. xylosoxidans bloodstream infections in the past 3 months. To identify the source of the outbreak and risk factors for infection and to implement control measures, ACDC then initiated an outbreak investigation.
The outbreak investigation focused on Office B. To identify all patients associated with Office B who had had a positive A. xylosoxidans culture, we requested a review of medical records and laboratory reports.
Matched Case–Control Study
A matched case–control study was performed to determine risk factors for infection. A case-patient was defined as a patient of Office B who had a positive A. xylosoxidans blood culture from November 2001 through January 2002. Controls were defined as patients who had no symptoms or signs of bloodstream infection (fever, chills, rigors, myalgias, nausea, vomiting, weakness, or hypotension). For each case-patient, 5–7 controls were randomly selected and matched by the closest date of their visits to Office B to the case-patient’s date of visit. Variables included age, sex, underlying diagnosis, intravenous medications received, peripheral white blood cell counts, presence and type of central venous catheter (CVC), clinic visits, hospitalization dates, symptoms, and types of invasive procedures. Data were collected on standardized forms and analyzed by using Epi Info 2000 version 1.1.2 (Centers for Disease Control and Prevention [CDC], Atlanta, GA, USA); odds ratios were used to estimate risk, t tests were performed for continuous variables, and p<0.05 indicated statistical significance.
Prospective Cohort Study
To identify possible A. xylosoxidans bloodstream infection, ACDC conducted prospective blood culture surveillance. On February 15, 2002, all patients with a CVC who had visited Office B since November 2001 were sent a letter requesting them to have a culture performed on blood drawn through the CVC. CVCs were removed from those whose culture results were positive.
Environmental Investigation
On January 17, 2002, numerous open containers (multidose 30-mL vials of heparin; 100-mL and 150-mL bottles of saline; and containers of alcohol, hydrogen peroxide, betadine, and iodine) were collected and sent to the Los Angeles County Public Health Laboratory for analysis. On February 15, 2002, environmental samples and swabs were collected for culture from work surfaces (e.g., countertops, sinks, hoods, kitchens) and from tap water and hands of healthcare workers who accessed CVCs, collected blood, prepared flushes, or administered chemotherapy.
Infection Control
During January and February 2002, we made several site visits to Office B to observe procedures, review medical records, and interview the office staff. Specifically, we observed procedures for preparation and administration of intravenous medications and asked office staff about level of technical training, experience, and license status.
Molecular Studies
Blood isolates of A. xylosoxidans from case-patients were obtained from Hospital A’s laboratory. For comparison, all A. xylosoxidans isolated from patients from Los Angeles County in the past 6 months were obtained from a large local reference laboratory. Pulsed-field gel electrophoresis (PFGE) was performed at the Los Angeles County Public Health Laboratory by using standard methods (18) for Salmonella spp. with the exception that isolates were digested with XbaI and SpeI. PFGE pattern comparisons were performed visually by using criteria established by Tenover et al. (19).
Examination of CVC for Biofilm
A CVC (PASport, a single-lumen, 6 French catheter with an under-the-skin titanium port; SIMS Deltec, Inc., St. Paul, MN, USA) was surgically removed from an asymptomatic patient identified in the prospective cohort study. Aseptic methods were used. The distal lumen opening was clamped with a sterile hemostat to retain the liquid within the lumen, and the catheter was placed in a sealed, sterile container and shipped overnight to CDC in Atlanta for processing within 24 hours of collection. At CDC, the CVC was placed into a Class II Biological Safety Cabinet, and a 1-cm segment was removed with a sterile scalpel. This segment was cut lengthwise to expose the lumen, and the individual pieces were placed into 5% glutaraldehyde (Ted Pella, Redding, CA, USA) in 0.67 M cacodylate buffer, pH 6.2, and processed for scanning electron microscopy (20). Samples were examined by using a Philips XL 20 Scanning Electron Microscope (FEI Company, a subsidiary of Philips, Hillsboro, OR, USA). The remaining catheter attached to the titanium port was clamped, and the outer surface was cleaned with a 70% alcohol wipe, disinfected, and processed to recover biofilm organisms (20). The recovered organisms were plated on trypticase soy agar containing 5% sheep blood (blood agar; Becton, Dickinson, Sparks, MD, USA). Plates were incubated for up to 72 h at 35°C and then examined. Colonies were spread onto blood agar for subculture and identified by using standard clinical microbiologic methods (21). Biofilm isolates were also characterized by PFGE (methods described above).
A total of 12 patients with A. xylosoxidans bloodstream infection were found: 9 from the retrospective case–control study and 3 from the prospective study (Table). All 12 were immunocompromised. Their ages ranged from 41 to 79 years (mean 65.8 years), and 10 (83.3%) were female. Case-patients had differing underlying diagnoses and chemotherapy regimens. Case-patients had had fevers, chills, and/or rigors within minutes to days after an infusion through their CVC. Several case-patients had multiple episodes of fever and chills during and immediately after visits to Office B when their CVC was accessed for blood collection, chemotherapy, or routine flushes. For some, these symptoms were attributed to possible side effects of chemotherapy. All case-patients had visited Office B from November 12 through December 18, 2001. Case-patient 1 was hospitalized from October 12 through November 10, 2001, and had visited Office B for daily collection to monitor neutropenia from November 13 through 19, 2001. Patients with A. xylosoxidans bloodstream infection were treated with antimicrobial drugs and CVC removal. Available records showed case-patients were treated with piperacillin/tazobactam; 1 case-patient who was allergic to penicillin was treated with aztreonam. One patient died from underlying malignancy (end-stage pancreatic cancer).
Matched Case–Control Study
Of the 9 case-patients identified, 7 who had clear onset date of bloodstream infection symptoms were selected for the case–control study. Case-patients were younger than controls (mean age 63.5 years [range 41–73 years] and mean age 73.2 years [range 35–89 years], respectively; p = 0.047). Case-patients were significantly more likely to have a CVC than controls. Matched case–control analysis showed that all 7 case-patients versus 4 of 47 control patients had a CVC at the time of illness onset (p<0.0001). The 2 other case-patients not included in the case–control study also had CVCs. Patients with CVCs received heparin and saline flushes before and after the CVC was used for blood collection or infusions. No records documented when each of the Office B nurses accessed the CVCs. Patients without CVCs who needed only blood collection for testing did not receive any flushes; however, those without CVCs who needed blood tests before receiving an infusion received a heparin and saline flush after a peripheral intravenous line was placed. Case-patients and controls did not have statistically significant differences in peripheral leukocyte counts, intravenous medications administered, types of chemotherapy received, or underlying diseases.
Prospective Cohort Study
In February 2002, 29 patients with CVCs had blood collected for culture. Of the 3 (10%) who had positive culture results for A. xylosoxidans, chart review showed that 2 had been intermittently symptomatic (Table).
Environmental Investigation
Cultures from available open solutions in the oncology office, collected 6 weeks after the initial cluster of A. xylosoxidans–positive blood cultures, and environmental cultures did not grow A. xylosoxidans. A sample from a sterile saline bottle that was open in the infusion room was positive for Bacillus circulans, and a tap water sample was positive for Moraxella spp.
Infection Control Practices and Procedures
Office B had 10 patient examination rooms and a separate, large, open infusion room where several patients could receive chemotherapy. The infusion room contained a hood and sink for preparation of intravenous medication. Of the 4 staff members at Office B who regularly accessed CVCs; inserted peripheral intravenous catheters; collected blood; and prepared or administered chemotherapy, flushes, or intravenous medications, only 1 was a registered nurse who had a California state license. The 3 nonlicensed staff members were reported to have received nursing training in their native country but did not have documented formal training or education. One nurse wore artificial fingernails but had removed them before hand culture samples were collected; thus, the fingernails were unavailable for culture. The following breaches in infection control were noted: intravenous catheters were inserted by persons not wearing gloves; unlabeled, prefilled syringes were stored in the hood; no documentation of hood cleaning was found; open, multidose heparin vials and saline bottles, some undated, were found throughout the facility; nonhygienic material was stored in the chemotherapy medication preparation hood; and failure to wash hands between patients was noted. No pharmacists were employed at Office B. No documentation of staff training and evaluation for chemotherapy preparation or infection control competency was available.
CVC Biofilm Studies
Scanning electron microscopy of the CVC showed a biofilm that contained rod-shaped bacteria in association with fibrinlike material on the catheter surface (Figure 1). A pure bacterial culture recovered from the CVC lumen was identified as A. xylosoxidans.
Molecular Studies
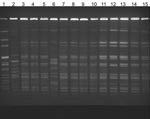
A. xylosoxidans blood culture isolates from case-patients were indistinguishable by PFGE analysis (Figure 2); in contrast, 3 A. xylosoxidans isolates from a local reference laboratory had different PFGE patterns. The isolate from the CVC biofilm matched the A. xylosoxidans bloodstream infection outbreak strain.
This large outbreak (N = 12) of A. xylosoxidans bloodstream infections was caused by 1 strain, which was also isolated from CVC biofilm. Symptoms of bloodstream infection probably occurred when flushes detached bacteria from the CVC biofilm. The prospective study found that 3 (10%) of 29 patients had A. xylosoxidans–positive blood cultures. Our case–control and prospective studies support the association of A. xylosoxidans bloodstream infection and CVCs, and our molecular biologic studies confirm A. xylosoxidans biofilm of the same outbreak strain on a CVC. A. xylosoxidans outbreaks reported to date have been associated with healthcare and contamination of hospital products (1,2,5,12–14), but none occurred in an outpatient setting.
The cause of this outbreak most likely was the use of contaminated multidose vials of heparin or saline flushes, leading to the formation of an A. xylosoxidans biofilm on CVCs. Case-patient 1 had been hospitalized from late October through early November at Hospital A. During November 13–19, 2001, this case-patient had had blood collected and her CVC line flushed numerous times at Office B; on November 19, culture result indicated A. xylosoxidans infection, which was successfully treated. We observed multiple breaches in infection control at Office B: use of unlabeled, prefilled syringes, poor hand hygiene, and lack of glove use. The CVC of case-patient 1 may have been flushed by using the same syringe and needle inserted into multidose vials, causing contamination of the vials. Another possible route of contamination is through artificial fingernails. A cluster of Serratia marcescens and A. xylosoxidans bacteremia cases linked to multidose heparin vials contaminated by a nurse with artificial fingernails has been reported (22); however, the artificial fingernails from the nurse at Office B were unavailable for testing. We suspect that multidose vials were contaminated with A. xylosoxidans and subsequently used on other patients from November 12 through December 18, 2001, when all case-patients had overlapping visits at Office B and received CVC flushes. A culture from an open, supposedly sterile saline bottle grew B. circulans, which suggests possible breaches in infection control. Multidose heparin and saline vials have been reported as the cause of outbreaks of hepatitis C (23,24), S. marcescens (25), and Pseudomonas aeruginosa (26) infections.
Although the heparin and the saline vials could have been contaminated from case-patient 1 in November, case-patients who subsequently received flushes from these vials may not have become immediately ill with symptoms of bloodstream infection. A. xylosoxidans biofilm could have developed on their CVCs and intermittently caused clinical illness when the CVCs were manipulated; i.e., flushing dislodged the biofilm and caused symptomatic bacteremia. Although indwelling catheters are frequently colonized with biofilm shortly after insertion (27), colonization does not necessarily lead to infection; bloodstream infection symptoms from an organism in contaminated intravenous solutions have been delayed for as long as 421 days after exposure (28). The finding of an asymptomatic patient with a CVC with A. xylosoxidans biofilm supports this. A number of variables may be associated with detachment of microbial cells from a biofilm (29), resulting in erosion or sloughing. Flushing, which could mechanically shear the biofilm, could result in detachment of cells or aggregates that could in turn colonize the bloodstream and cause signs and symptoms of bacteremia. This phenomenon has been recently reported (28).
The case-patients in this outbreak had their CVCs removed and were treated with antimicrobial agents. The presence of A. xylosoxidans biofilm and the mechanism of bloodstream infection after disruption by catheter flushing suggests that eradication of infection would require catheter removal, as reported by others (4,9). Previously, recurrent A. xylosoxidans bacteremia has been reported in those patients whose indwelling catheters were not removed (11). Formation of A. xylosoxidans biofilm provides an explanation for the organism most commonly causing bacteremia in patients with CVCs (7,10).
The California Code of Regulations, Title 17, Section 2500 (30), requires all healthcare professionals to immediately report outbreaks of any cause; however, this outbreak was not recognized early on. The initial cluster of patients at Office B had symptoms and positive blood cultures growing this uncommon organism for 6 weeks before the cases were reported to the Los Angeles County Department of Public Health. Because outpatient settings may lack surveillance systems, outbreaks may not be recognized immediately, thus potentially exposing more patients. In addition, some of the symptoms of bloodstream infection were initially attributed to side effects of chemotherapy. Because 10% of patients in our prospective cohort study had blood cultures positive for A. xylosoxidans, further studies are needed to determine whether active surveillance for patients with CVCs would help recognize infections.
Because we noted not only infection control breaches but also that unlicensed office personnel were manipulating the CVCs, line flushes, infusions, and blood collection through CVCs, we reported the situation to the California Medical Board and the California Department of Health Services. Although no California state regulations for infection control in outpatient physician’s offices exist, the California Department of Health Services and Los Angeles County Department of Public Health recommended that the oncology office improve infection control standards; handling, storage, exposure, and access to pharmaceuticals; and improve medical record documentation. New infection control policies were established, and the office subsequently hired new, properly licensed registered nurses and nurse practitioners to handle insertion of intravenous catheters and administration of intravenous medications and chemotherapy. After proper education of the oncology office staff and removal of multidose vials of heparin and saline, no more A. xylosoxidans bloodstream infections were reported from Office B.
Our investigation has limitations. We did not culture A. xylosoxidans from the multidose vials. The original vials, used when the outbreak began, had already been discarded and were not available for testing by the time we were notified in January 2002. Our investigation was also limited by the absence of medical records indicating when nursing staff accessed the CVCs. Although the contamination of multidose vials remains suggestive, we suspect that they were the most likely source. The outbreak ended after discontinuing their use and enacting improved infection control practices. The organism could have been introduced into multidose vials by a needle or syringe used on an infected patient or by the artificial fingernails of the nurse, through gaps in infection control.
For patients who received infusion therapy at home, receipt of therapy in an outpatient clinic or physician’s office was an independent risk factor for bloodstream infection (31). Therefore, clinicians need to be vigilant because minor breaches in infection control can lead to large outbreaks with uncommon human pathogens, especially in patients with CVCs. Clinicians also need to ensure that appropriate infection control practices are adhered to consistently, especially in outpatient care settings, where oversight of infection control procedures may be absent. Unlike standards that exist for nursing homes or hospitals, no written standards regarding infection control in outpatient settings exist from the California Department of Health Services or the California Medical Board. However, routine monitoring and adherence to infection control could prevent outbreaks. Clinicians providing care in outpatient settings should review appropriate infection control standards and consider establishing written policies to ensure that standards are met. As healthcare delivery continues to move toward outpatient care (32), the lack of formal infection control procedures and accountability in the outpatient office setting can lead to large disease outbreaks (33,34); the need for oversight in this setting should be considered.
Our investigation helps characterize the mechanisms of A. xylosoxidans bloodstream infection in immunocompromised patients with CVCs. It provides a better understanding of how biofilm formation in a CVC can result in a clinical infectious disease process with this uncommon organism. Substantial illness and death can occur in outpatient settings that lack formal oversight. This outbreak highlights an unaddressed infection control problem in the outpatient setting for regulating agencies to further review.
Dr Kim is an infectious disease physician at the Acute Communicable Disease Control Program, Department of Public Health, in Los Angeles County. Her research interests focus on the public health effects of infectious pathogens.
Acknowledgment
We thank Ricardo Murga, Janice Carr, Bette Jensen, and Phillip K. Ng for their contributions during this outbreak investigation.
References
- Reverdy ME, Freney J, Fleurette J, Coulet M, Surgot M, Marmet D, Nosocomial colonization and infection by Achromobacter xylosoxidans. J Clin Microbiol. 1984;19:140–3.PubMedGoogle Scholar
- Gahrn-Hansen B, Alstrup P, Dessau R, Fuursted K, Knudsen A, Olsen H, Outbreak of infection with Achromobacter xylosoxidans from contaminated intravascular pressure transducers. J Hosp Infect. 1988;12:1–6. DOIPubMedGoogle Scholar
- Duggan JM, Goldstein SJ, Chenoweth CE, Kauffman CA, Bradley SF. Achromobacter xylosoxidans bacteremia: report of four cases and review of the literature. Clin Infect Dis. 1996;23:569–76.PubMedGoogle Scholar
- Knippschild M, Schmid EN, Uppenkamp M, König E, Meusers P, Brittinger G, Infection by Alcaligenes xylosoxidans subsp. xylosoxidans in neutropenic patients. Oncology. 1996;53:258–62.PubMedGoogle Scholar
- Vu-Thien H, Darbord JC, Moissenet D, Dulot C, Dufourcq JB, Marsol P, Investigation of an outbreak of wound infections due to Alcaligenes xylosoxidans transmitted by chlorhexidine in a burns unit. Eur J Clin Microbiol Infect Dis. 1998;17:724–6. DOIPubMedGoogle Scholar
- Gómez-Cerezo J, Suárez I, Ríos JJ, Peña P, García de Miguel MJ, de José M, Achromobacter xylosoxidans bacteremia: a 10-year analysis of 54 cases. Eur J Clin Microbiol Infect Dis. 2003;22:360–3. DOIPubMedGoogle Scholar
- Tsay RW, Lin LC, Chiou CS, Liao JC, Chen CH, Liu CE, Alcaligenes xylosoxidans bacteremia: clinical features and microbiological characteristic of isolates. J Microbiol Immunol Infect. 2005;38:194–9.PubMedGoogle Scholar
- Legrand C, Anaissie E. Bacteremia due to Achromobacter xylosoxidans in patients with cancer. Clin Infect Dis. 1992;14:479–84.PubMedGoogle Scholar
- Hernandez JA, Martino R, Pericas R, Sureda A, Brunet S, Domingo-Albos A. Achromobacter xylosoxidans bacteremia in patient with hematologic malignancies. Haematologica. 1998;83:284–5.PubMedGoogle Scholar
- Aisenberg G, Rolston KV, Safdar A. Bacteremia caused by Achromobacter and Alcaligenes species in 46 patients with cancer (1989–2003). Cancer. 2004;101:2134–40. DOIPubMedGoogle Scholar
- Shie SS, Huang CT, Leu HS. Characteristics of Achromobacter xylosoxidans bacteremia in northern Taiwan. J Microbiol Immunol Infect. 2005;38:277–82.PubMedGoogle Scholar
- Reina J, Antich M, Siquier B, Alomar P. Nosocomial outbreak of Achromobacter xylosoxidans associated with a diagnostic contrast solution. J Clin Pathol. 1988;41:920–1. DOIPubMedGoogle Scholar
- Lehours P, Rogues AM, Occhialini A, Boulestreau H, Gachie JP, Megraud F. Investigation of an outbreak due to Alcaligenes xylosoxidans subspecies xylosoxidans by random amplified polymorphic DNA analysis. Eur J Clin Microbiol Infect Dis. 2002;21:108–13. DOIPubMedGoogle Scholar
- Tena D, Carranza R, Barberá JR, Valdezate S, Garrancho JM, Arranz M, Outbreak of long-term intravascular catheter-related bacteremia due to Achromobacter xylosoxidans subspecies xylosoxidans in a hemodialysis unit. Eur J Clin Microbiol Infect Dis. 2005;24:727–32. DOIPubMedGoogle Scholar
- McGuckin MB, Thorpe RJ, Koch KM, Alavi A, Staum M, Abrutyn E. An outbreak of Achromobacter xylosoxidans related to diagnostic tracer procedures. Am J Epidemiol. 1982;115:785–93.PubMedGoogle Scholar
- Granowitz EV, Keenholtz SL. A pseudoepidemic of Alcaligenes xylosoxidans attributable to contaminated saline. Am J Infect Control. 1998;26:146–8. DOIPubMedGoogle Scholar
- Kim MJ, Bancroft E, Mascola L, Lehnkering E, Lawani L. Outbreak of Alcaligenes xylosoxidans bloodstream infections in an outpatient oncology office, Los Angeles, 2002 [abstract 415]. In: Program and abstracts of the 40th annual meeting of the Infectious Diseases Society of America (Chicago). Alexandria (VA): Infectious Disease Society of America; 2002.
- Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. DOIPubMedGoogle Scholar
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.PubMedGoogle Scholar
- Donlan RM, Murga R, Bell M, Toscano CM, Carr JH, Novicki TJ, Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J Clin Microbiol. 2001;39:750–3. DOIPubMedGoogle Scholar
- Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, eds. Manual of clinical microbiology. 7th ed. Washington: American Society for Microbiology; 1999.
- Gordin FM, Schultz ME, Huber R, Zubairi S, Stock F, Kariyil J. A cluster of hemodialysis-related bacteremia linked to artificial fingernails. Infect Control Hosp Epidemiol. 2007;28:743–4. DOIPubMedGoogle Scholar
- Krause G, Trepka MJ, Whisenhunt RS, Katz D, Nainan O, Wiersma ST, Nosocomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol. 2003;24:122–7. DOIPubMedGoogle Scholar
- Bruguera M, Saiz JC, Franco S, Giménez-Barcons M, Sánchez-Tapias JM, Fabregas S, Outbreak of nosocomial hepatitis C virus infection resolved by genetic analysis of HCV RNA. J Clin Microbiol. 2002;40:4363–6. DOIPubMedGoogle Scholar
- Tanaka T, Takahashi H, Kobayashi JM, Ohyama T, Okabe N. A nosocomial outbreak of febrile bloodstream infection caused by heparinized-saline contaminated with Serratia marcescens, Tokyo, 2002. Jpn J Infect Dis. 2004;57:189–92.PubMedGoogle Scholar
- Prospero E, Barbadoro P, Savini S, Manso E, Annino I, D’Errico MM. Cluster of Pseudomonas aeruginosa catheter-related bloodstream infections traced to contaminated multidose heparinized saline solutions in a medical ward.. Int J Hyg Environ Health. 2006;209:553–6. DOIPubMedGoogle Scholar
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: delayed onset Pseudomonas fluorescens bloodstream infections after exposure to contaminated heparin flush—Michigan and South Dakota, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55:961–3.PubMedGoogle Scholar
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90.PubMedGoogle Scholar
- California Code of Regulations. Title 17, Section 2500 [cited 2007 Jun 22]. Available from http://ccr.oal.ca.gov/linkedslice/default.asp?SP=CCR-1000&Action=Welcome
- Tokars JI, Cookson ST, McArthur MA, Boyer CL, McGeer AJ, Jarvis WR. Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy. Ann Intern Med. 1999;131:340–7.PubMedGoogle Scholar
- Jarvis WR. Infection control and changing health-care deliver systems. Emerg Infect Dis. 2001;7:170–3.PubMedGoogle Scholar
- Samandari T, Malakamadze N, Balter S, Perz JF, Khristova M, Swetnam L, A large outbreak of hepatitis B virus infections associated with frequent injections at a physician’s office. Infect Control Hosp Epidemiol. 2005;26:745–50. DOIPubMedGoogle Scholar
- Watson JT, Jones RC, Siston AM, Fernandez JR, Martin K, Beck E, Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch Intern Med. 2005;165:2639–43. DOIPubMedGoogle Scholar
Figures
Table
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to http://www.medscape.com/cme/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.com. If you are not registered on Medscape.com, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Alcaligenes xylosoxidans Bloodstream Infections in Outpatient Oncology Office
CME Questions
1. Which of the following statements about Alcaligenes xylosoxidans is most accurate?
A. A. xylosoxidans is gram-positive
B. A. xylosoxidans is hydrophobic
C. A. xylosoxidans is associated with healthcare-associated infections
D. A. xylosoxidans is found only in soil
2. Which of the following statements about the clinical presentation of A xylosoxidans infection in the current study is most accurate?
A. No patients with A. xylosoxidans infection were immunocompromised
B. Patients with A. xylosoxidans were homogeneous with regard to age and clinical diagnosis
C. Patients with A. xylosoxidans infection had symptoms that were attributed to side effects of chemotherapy
D. Only 10% of patients with A. xylosoxidans infection were successfully treated with antibiotics
3. Which of the following factors was most different in comparing case-patients and control groups in the current study?
A. Case-patients with A. xylosoxidans infection had a lower mean white blood cell count compared with controls
B. Case-patients with A. xylosoxidans infection were more likely to have a central venous catheter compared with controls
C. Case-patients with A. xylosoxidans infection had a greater number of intravenous medications administered compared with controls
D. Case-patients with A. xylosoxidans infection had more underlying diseases compared with controls
4. Which of the following sources was most likely the primary reservoir of A. xylosoxidans in the current study?
A. Tap water
B. Mixing hood
C. Hospital personnel
D. Multidose vials of heparin and saline flushes
Activity Evaluation
| 1. The activity supported the learning objectives. | ||||
| Strongly Disagree | Strongly Agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly Disagree | Strongly Agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly Disagree | Strongly Agree | |||
| 1 | 2 | 3 | 4 | 5 |
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly Disagree | Strongly Agree | |||
| 1 | 2 | 3 | 4 | 5 |
Related Links
Table of Contents – Volume 14, Number 7—July 2008
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Moon J. Kim, Acute Communicable Disease Control Program, Los Angeles County Department of Public Health, 313 N Figueroa St, Rm 222, Los Angeles, CA 90012, USA;
Top