Volume 15, Number 11—November 2009
Dispatch
Imported Melioidosis, Israel, 2008
Cite This Article
Citation for Media
Abstract
In 2008, melioidosis was diagnosed in an agricultural worker from Thailand in the southern Jordan Valley in Israel. He had newly diagnosed diabetes mellitus, fever, multiple abscesses, and osteomyelitis. Burkholderia pseudomallei was isolated from urine and blood. Four of 10 laboratory staff members exposed to the organism received chemoprophylaxis, 3 of whom had adverse events.
Melioidosis, which is caused by Burkholderia pseudomallei, is endemic to some areas of Southeast Asia and northern Australia (1,2). Recent data indicate that it is now endemic to most of the Indian subcontinent, southern People’s Republic of China, Hong Kong, Taiwan, Papua New Guinea, and other regions (3). Most cases reported in other regions were acquired during residence in or travel to disease-endemic regions.
Thailand is a popular destination for backpackers from Israel. Importation of melioidosis has long been anticipated as a potential problem because many persons from Thailand are employed in Israel. Although most infections are asymptomatic (2) and usually occur in persons <6 years of age in disease-endemic areas, clinical, often life-threatening, disease most frequently affects adults who have underlying predisposing conditions, especially type 2 diabetes. Incubation period differs according to manner of exposure and size of inoculum and may be short (1 day to 2–3 weeks). However, because the organism has a proclivity for latency (4), the disease may appear after months or many years (2,4). Melioidosis is often manifested as pneumonia, but its hallmark is disseminated abscess formation in viscera, skin, soft tissue, and bone. We report a case of imported melioidosis (5) and management and consequences of chemoprophylaxis among laboratory staff exposed to B. pseudomallei.
A 32-year-old man from Thailand was referred to the emergency department of Hadassah–Hebrew University Hospital at Mount Scopus on July 31, 2008, with newly diagnosed diabetes and fever. He reported 2–3 weeks of fatigue, chills, night sweats, and a weight loss of ≈25 kg in the past 2 months. Two large subcutaneous abscesses had developed over the past several months. The first abscess, in the right axilla, had been drained in May 2008. The second abscess, in the upper right abdominal wall, had been drained in July 2008. Pus was not submitted for culture.
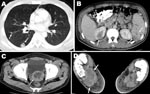
The patient, an agricultural worker, arrived in Israel in November 2007 and was employed at a rural settlement in the southern Jordan Valley. He came from a village in northeastern Thailand where he had worked in rice and sugar cane farming. At the time of admission, he appeared ill and was febrile (39.0°C). Physical examination showed mild cervical lymphadenopathy, nontender hepatomegaly, and healing wounds from the abscess drainage procedures. Laboratory results showed hyperglycemia (glucose level 19.4 mmol/L), normocytic anemia with normal leukocyte and platelet counts, an erythrocyte sedimentation rate of 105 mm/h, and moderately elevated levels of alkaline phosphatase and γ-glutamyl transferase. Kidney function was normal. Urinalysis showed leukocyturia and nitrites. Multiple abscesses were seen in the spleen, lungs, superior pole of the right kidney, prostate gland, and right foot (Figure). A bone scan confirmed osteomyelitis of the right medial malleolus, calcaneus, and first metatarsus.
A diagnosis of melioidosis had been considered from the outset in view of clinical findings of multiple abscesses in a patient with diabetes from a disease-endemic area. A blood culture (BacTec+ Aerobic/F; Becton, Dickinson and Company, Sparks, MD, USA) yielded a nonfermentative, oxidase-positive, motile, colistin-resistant, gram-negative bacilli that showed dry wrinkled colonies. The API 20 NE profile (API; bioMérieux, Durham, NC, USA) was 1556577, which suggested esculin-positive B. pseudomallei.
Molecular confirmation was achieved by bidirectional sequencing of a 1.7-kb amplicon specific for the 16S rRNA gene, which was amplified by PCR and primers F229 and R1908 (6). Sequencing (National Center for Biotechnology Information accession no. FJ426359) with the same primers showed the known single basepair transition (C/T) at position 75 that distinguishes B. pseudomallei from B. mallei, the agent of glanders (6).
Susceptibility to trimethoprim/sulfamethoxazole was confirmed. MICs were 0.75 mg/L for trimethoprim and 14.25 mg/L for sulfamethoxazole (Etest; AB Biodisk, Solna, Sweden). A urine culture was positive for B. pseudomallei, and throat and splenic abscess aspirate cultures were negative.
The patient’s first abscess developed ≈6 months after his arrival in Israel, which attests to a long incubation period or prolonged latency after initial asymptomatic infection. New-onset diabetes might have unmasked a preexisting latent infection. He had no history of an illness compatible with melioidosis before he left Thailand.
Treatment with ceftazidime (2 g intravenously 4×/d) and trimethoprim/sulfamethoxazole (1,920 mg orally 2×/d) for 4 weeks resulted in gradual defervescence. The patient was discharged with instructions to take trrimethoprim/sulfamethoxazole (1,920 mg orally 2×/d) and doxycycline (100 mg orally 2×/d) for an additional 20 weeks. He returned to Thailand a few weeks after discharge.
B. pseudomallei infection has been regarded as an occupational hazard for clinical microbiologists (7–9). Although risk for laboratory-acquired infection is relatively low (7), the nature of the disease demands special precautions in dealing with its causative organism. With recent designation of B. pseudomallei as a select agent by the Centers for Disease Control and Prevention (www.cdc.gov/od/sap), it has been proposed that Biosafety Level 2 practices, which were advised for clinical diagnostic work (10), be replaced by stricter safety practices (11).
Accordingly, a risk assessment was performed, and 4 persons who had handled the cultures outside a biologic safety cabinet were offered postexposure chemoprophylaxis with trimethoprim/sulfamethoxazole (1,920 mg orally every 8 h) for 3 weeks) (11). The frequency of rashes was worrisome: rashes developed in 2 persons (1 elected to complete the course of doxycycline [100 mg orally 2×/d for 3 weeks] and 1 stopped treatment after 10 days). One person was so uncomfortable that she refused further treatment on day 12. Only 1 person completed the course of trimethoprim/sulfamethoxazole without adverse effects. Symptoms consistent with melioidosis did not develop in any of the exposed staff. Serum samples from 10 staff members who had had any contact with the cultures were collected within 2–3 days of exposure and after 6–8 weeks and tested by using an indirect hemagglutination test at Mahidol University in Bangkok. All serologic test results were negative, an outcome consistent with findings of a published report (8).
Many workers (10,600 in 2007) from Thailand have been employed in agriculture in Israel (12). Cases of melioidosis may not have been detected until the present patient because routine preemployment medical screenings may have excluded persons with the disease from entry into Israel or unfamiliarity with the disease led to underdiagnosis.
Migration of populations requires awareness regarding unforeseen diseases, as recently highlighted in a clinical problem-solving exercise (13). If one considers the abscesses in our patient, melioidosis would have likely been diagnosed earlier had this patient remained in Thailand. In any case, if the routine practice of culturing pus from his abscesses had been followed, the diagnosis might have been made earlier. This finding is a reminder to physicians that they should adhere to basic clinical guidelines. Conversely, clinical evidence and close cooperation of ward physicians, the infectious disease service, and the laboratory staff likely expedited identification of the organism.
The diagnosis of melioidosis in a region where this disease is not endemic depends on physician awareness and laboratory capability. In the patient reported here, clinical suspicion, suggested by multiple visceral abscesses, preceded microbiologic confirmation, which was expedited by internists and the infectious disease service. Clinical microbiology laboratories worldwide should prepare for dealing with B. pseudomallei and include it in the workup of unusual nonfermentative, colistin-resistant, gram-negative bacilli, particularly in workers from Thailand or other melioidosis-endemic countries. The issue of chemoprophylaxis for persons with laboratory exposures, and its potential for adverse events, requires careful immediate decision making, especially if one considers the rarity of this albeit disabling disease among laboratory staff.
Dr Cahn is a senior resident in Internal Medicine at the Hadassah–Hebrew University Hospital, Mount Scopus, Jerusalem. Her research interests include diabetes and diabetic foot infections.
Acknowledgment
We thank Sharon J. Peacock, Vanaporn (Lek) Wuthiekanun, and their staff for performing the serologic tests.
References
- Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. DOIPubMedGoogle Scholar
- Currie BJ. Burkholderia pseudomallei and Burkholderia mallei: melioidosis and glanders. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases, 6th ed. Philadelphia: Churchill Livingstone; 2005. p. 2622–30.
- Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S1–4. DOIPubMedGoogle Scholar
- Gan YH. Interaction between Burkholderia pseudomallei and the host immune response: sleeping with the enemy? J Infect Dis. 2005;192:1845–50. DOIPubMedGoogle Scholar
- Block C. Melioidosis—Israel ex Thailand. ProMed. August 15, 2008 [cited 2009 Jul 15]. Available from http://www.promedmail.org, archive number 20080819.2588.
- Gee JE, Sacchi CT, Glass MB, De BK, Weyant RS, Levett PN, Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J Clin Microbiol. 2003;41:4647–54. DOIPubMedGoogle Scholar
- Ashdown LR. Melioidosis and safety in the clinical laboratory. J Hosp Infect. 1992;21:301–6. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Laboratory exposure to Burkholderia pseudomallei—Los Angeles, California, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:988–90.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Imported melioidosis—South Florida, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:873–6.PubMedGoogle Scholar
- Chosewood LC, Wilson DE, eds. Biosafety in microbiological and biomedical laboratories, 5th ed. Washington: US Department of Health and Human Services, US Government Printing Office; 2007 [cited 2009 Jul 15]. Available from http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm
- Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V, Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg Infect Dis. 2008 [cited 2009 Jul 15]. Available from http://www.cdc.gov/EID/content/14/7/e2.htm
- Central Bureau of Statistics. Statistical abstract of Israel, 2008, no. 59, table 4.11. Tel Aviv (Israel): The Bureau [cited 2009 Jul 16]. Available from http://www.cbs.gov.il/shnaton59/download/st04_11.xls
- Falade OO, Antonarakis ES, Kaul DR, Saint S, Murphy PA. Beware of first impressions. N Engl J Med. 2008;359:628–34. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 15, Number 11—November 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Colin Block, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, PO Box 12000, Ein Kerem, Jerusalem 91120, Israel
Top