Volume 15, Number 2—February 2009
Letter
Human Case of Atopobium rimae Bacteremia
To the Editor: The genus Atopobium (1) accommodates species formerly designated Lactobacillus minutus, L. rimae, and Streptococcus parvulus (2). Use of 16S rDNA sequence analysis showed these species to be closely related and to form a distinct line of descent within the lactic acid bacteria (3). Atopobium spp. usually have been recognized as part of the human gingival oral flora; some species, including A. rimae and A. parvulum, have been identified as agents of chronic periodontitis (4,5). A. rimae, formerly known as L. rimae (1), forms short, gram-positive, strictly anaerobic, elliptical bacteria with low G+C content (4). A. rimae produces large amounts of lactic acid and has been recovered previously from normal human gingival flora (4,5). Apart from periodontitis, it has not been implicated in other types of infection. We report an unusual case of A. rimae bacteremia.
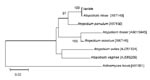
In May 2007, a 77-year-old woman with a history of right thoracotomy for pneumothorax 2 years earlier was hospitalized for inhalation pneumonia caused by paralysis of the right vocal cord. During hospitalization, septic shock and a fever of 38°C developed in the patient, complicated by acute respiratory failure and stroke. She was transferred to an intensive care unit with a PaO2/FiO2 >300 mm Hg, and a tracheotomy was performed. Three anaerobic blood specimens, drawn at entrance into the intensive care unit, yielded gram-positive cocci after 24-h incubation of the first bottle and gram-positive bacilli after 48-h incubation of the 2 other bottles. The gram-positive cocci were identified as Streptococcus gordonii using API STREP (bioMérieux, Marcy l’Etoile, France). The gram-positive bacilli were catalase negative and oxidase positive but remained unidentified with use of API ANA strip (bioMérieux). Minimum inhibitory concentrations of antibiotics were determined for the gram-positive bacilli using E-test assay (AB BIODISK, Solna, Sweden) on Columbia agar supplemented with 5% sheep blood. Minimum inhibitory concentrations were 0.064 μg/mL for penicillin G, 0.023 μg/mL for ampicillin, 0.012 μg/mL for amoxicillin–clavulanic acid, 0.032 μg/mL for imipenem, <0.016 μg/mL for azithromycin, <0.016 μg/mL for erythromycin, 0.06 μg/mL for ciprofloxacin, and 1.25 μg/mL for vancomycin. DNA was extracted from 1 colony by using a QIAamp tissue kit (QIAGEN, Hilden, Germany) as described by the manufacturer. The 1,454-bp 16S rDNA sequence obtained using the fD1 5′-AGAGTTTGATCCTGGCTCAG-3′ and rP2 5′-ACGGCTACCTTGTTACGACTT-3′ primer pair (6,7) showed 99% sequence similarity with the 16S rDNA sequence of A. rimae (GenBank accession no. AF292371) by use of BLAST version 2.2.9 software (National Center for Biotechnology Information). A phylogenetic neighbor-joining tree based on the Atopobium spp. 16S rDNA sequences made with the MEGA software confirmed that the isolate belonged to A. rimae (Figure). Initial treatment by intravenous tazocilline-amikacin was changed to intravenous amoxicillin–clavulanic acid (2 g/200 mg). The fever resolved, and the patient’s condition improved. The treatment was stopped after 7 days, and the patient remained apyretic.
In this case, phenotypic identification of gram-positive bacillus isolated from 2 blood cultures failed because the definite bacterial species A. rimae was not included in the API database used for the phenotypic identification. Final identification was achieved within 2 days by comparison of the almost complete 16S rDNA sequence with homologous sequences deposited in Genbank. This comparison yielded a 99% sequence similarity, regarded as criteria for accurate identification of bacterial organisms at the species level (8). In this patient, 2 A. rimae isolates were recovered from 2 different blood-culture bottles drawn 48 h apart, suggesting that A. rimae was not just a bypassing organism but indeed responsible for septicemia. In these specimens, S. gordonii was also isolated. Both species have been described as belonging to the oral flora, suggesting that these flora probably were the source for mixed septicemia in the patient. A. rimae was isolated as the patient was presenting with clinical features of septic shock, suggesting that A. rimae may have contributed to the shock. Antimicrobial drug treatment based on in vitro A. rimae susceptibility profile, along with reanimation measures, allowed for the patient’s recovery.
This case report illustrates the usefulness of 16S rDNA sequencing for accurate identification of anaerobic organisms and suggests that A. rimae should be added to the list of organisms responsible for bacteremia in patients.
References
- Collins MD, Wallbanks S. Comparative sequence analyses of the 16S rRNA genes of Lactobacillus minutus, Lactobacillus rimae and Streptococcus parvulus: proposal for the creation of a new genus Atopobium. FEMS Microbiol Lett. 1992;74:235–40.PubMedGoogle Scholar
- Rodriguez JM, Collins MD, Sjöden B, Falsen E. Characterization of a novel Atopobium isolate from the human vagina: description of Atopobium vaginae sp. nov. Int J Syst Bacteriol. 1999;49:1573–6.PubMedGoogle Scholar
- Dewhirst FE, Paster BJ, Tzellas N, Coleman B, Downes J, Spratt DA. Characterization of novel human oral isolates and cloned 16S rDNA sequences that fall in the family Coriobacteriaceae: description of Olsenella gen. nov., reclassification of Lactobacillus uli as Olsenella uli comb. nov. and description of Olsenella profusa sp. nov. Int J Syst Evol Microbiol. 2001;51:1797–804.PubMedGoogle Scholar
- Olsen I, Johnson JL, Moore LV, Moore WE. Lactobacillus uli sp. nov. and Lactobacillus rimae sp. nov. from the human gingival crevice and emended descriptions of lactobacillus minutus and Streptococcus parvulus. Int J Syst Bacteriol. 1991;41:261–6.PubMedGoogle Scholar
- Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–44. DOIPubMedGoogle Scholar
- Woo PC, Ng KH, Lau SK, Yip KT, Fung AM, Leung KW, Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J Clin Microbiol. 2003;41:1996–2001. DOIPubMedGoogle Scholar
- Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–55. DOIPubMedGoogle Scholar
- Drancourt M, Berger P, Raoult D. Systematic 16S rRNA gene sequencing of atypical clinical isolates identified 27 new bacterial species associated with humans. J Clin Microbiol. 2004;42:2197–202. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 15, Number 2—February 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michel Drancourt, Unité des Rickettsies, CNRS UMR 6020, IFR 48, Faculté de Médecine, Université de la Méditerranée, 27 Bd Jean Moulin, 13385 Marseille CEDEX 05, France; email:
Top