Volume 16, Number 1—January 2010
Dispatch
Reemergence of Syphilis in Martinique, 2001–2008
Cite This Article
Citation for Media
Abstract
Syphilis reemerged in Martinique in 2004 and initially affected 3 HIV-infected patients. By March 2008, syphilis was diagnosed for 37 men and 18 women. As of October 31, 2009, this outbreak had not yet been brought under control. It initially affected mainly men who had sex with men before it spread to heterosexual persons, minority group members, and crack cocaine users.
Syphilis was expected to reemerge in Martinique after outbreaks occurred in large western urban centers in 1998 (1,2), and cases were reported in Guadeloupe in 2001 (3). Soon after the first cases were diagnosed in Martinique, we conducted a study to determine whether these cases represented an outbreak and to identify demographic and social determinants (4) of this outbreak.
In 2001, we increased syphilis screening at University Hospital in Fort-de-France, Martinique. Screening included use of the rapid plasma reagin (RPR) test and the Treponema pallidum hemaglutination assay (TPHA). All positive and discordant results were verified by using fluorescent treponemal antibody absorption, which detects T. pallidum–specific immunoglobulin (Ig) G and IgM. Darkfield microscopy was used whenever possible. We reviewed medical files of all patients who had received a diagnosis of syphilis during January 1, 2001–March, 31, 2008. Patients were included in the study if they had recent syphilis (primary, secondary, or early latent stage) as defined by the US Centers for Disease Control and Prevention (Atlanta, GA, USA) (5).
We investigated the yearly incidence of recent syphilis among HIV-infected patients treated at the infectious diseases unit of the hospital, at the Vernes Sexually Transmitted Disease (STD) Clinic (Fort-de-France, Martinique), and at anonymous voluntary counseling and testing clinics. We also obtained syphilis test results of all persons who were tested at the central laboratory of the hospital. Laboratory definition of active syphilis was an RPR titer >4 and a TPHA titer >80 for an initial screening test, or a 4-fold increase in RPR titer in samples after previously positive results. TPHA screening results for voluntary blood donors were collected at a blood bank.
Recent syphilis was diagnosed for 55 patients at University Hospital during 2001–2008 (Table 1). Patients (37 men and 18 women) had a median age of 41 years (interquartile range [IQR] 36–44 years). Twenty-one (57%) of 37 men were men who have sex with men (MSM), and 9 (43%) of 21 were bisexual. One fourth of the patients never used condoms. Of 36 patients questioned about oral sex, 30 admitted practicing oral sex, of whom only 2 (6.6%) always used condoms. Each patient’s median number of sexual partners during the previous 12 months was 2.5 (IQR 1.5–3.5).
One of the first patients to receive a diagnosis in 2004 reported >100 sexual partners, most during a recent stay in Paris. Primary, secondary, and early latent syphilis was diagnosed in 12, 33, and 10 patients, respectively, and 21 patients with secondary syphilis had genital lesions. Cholestatic hepatitis developed in 7 of 29 HIV-positive patients. Six patients had neurosyphilis or ophthalmic syphilis, all of whom also had secondary rashes.
Median RPR titer for the 55 patients was 32. Results of darkfield microscopy were positive for 17 (74%) of 23 patient specimens, 5 from genital mucosa and 12 from skin lesions. All patients had prevention counseling and were successfully treated with penicillin (except for 1 patient who was successfully treated with azithromycin). Seven relapses occurred. More than half of the patients were HIV positive (53%): 22 with a previous diagnosis of HIV infection and 7 with a new diagnosis of HIV infection at the time of syphilis diagnosis. Median duration of HIV infection was 48 months (IQR 21–91 months), and median CD4 lymphocyte count was 516 cells/μL (IQR 340–639 cells/μL).
At the time of syphilis diagnosis, 8 patients were receiving highly active antiretroviral therapy, and 4 had an HIV viral load <50 copies/mL. Twenty-three patients (42%) were crack cocaine users, and 17 patients (7 heterosexual men, 2 MSM, and 8 women) (31%) lived in precarious conditions (defined as >1 of the following: homelessness, lack of welfare, being followed-up in a psychiatry unit, mental deficiency, having paid sex, incarceration in a correctional facility >2× in the past 5 years).
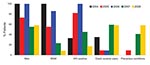
The first cases of syphilis were diagnosed in 2004, with peaks in number of cases in 2005 and 2007–2008 (Figure 1). Demographic and social characteristics of patients changed rapidly. The first peak in 2005 included mostly HIV-infected MSM, and the second peak in 2007–2008 included almost as many women as men and a larger proportion of persons living in precarious conditions; crack-cocaine use and paid sex were more frequent, but HIV infection was lower (Figure 2).
During 2000–2007, the incidence of syphilis among HIV-infected patients increased from 0/1,000 to 12.1/1,000 patient-years (Table 2). A marked decrease in cases of recent syphilis diagnosed at the STD clinic occurred during 1987–1992, and <5 cases were recorded yearly during 2001–2007. Among persons tested for syphilis in anonymous voluntary counseling and testing clinics, 12 were diagnosed with recent syphilis during 2002–2007 (incidence <5 cases/year).
Among blood donors, incidence of positive TPHA results was 0.3%–0.6% during 2000–2007, and an incidence of 1.04% was observed in 1999. However, the outbreak was too limited to have affected this group. The incidence of active syphilis as a proportion of all cases reported to the central laboratory database increased from 1.6% to 11.6% during 2000–2007 (Table 2).
Infectious syphilis had become so rare in France and in the French Caribbean islands in the 1990s that mandatory notification of this disease was canceled in 2000. That same year, outbreaks were reported by several STD clinics in France (6). These outbreaks followed reports of outbreaks among MSM in major urban centers in the United States (2,7), Canada, Europe, Australia, and New Zealand (4). Three epidemiologic profiles of syphilis patients were recently defined on the basis of social determinants (4): general populations in developing countries, minority populations with a low socioeconomic status in industrialized countries, and MSM.
In 2001, an outbreak of 58 cases of recent syphilis diagnosed during 1993–2001 (38 cases in 2001) was reported in Guadeloupe (3). This outbreak occurred mainly (89%) in the population living in precarious social and economic conditions; 21% had a history of recent imprisonment, and 50% used crack cocaine and had paid sex. The M:F ratio increased from 0.81:1 to 1.37:1. Twenty-six percent of the patients were HIV positive.
A limited but uncontrolled syphilis outbreak is ongoing in Martinique. It started with an MSM epidemiologic profile, then shifted to persons within a specific heterosexual group that included crack cocaine users. This shift is similar to that reported for HIV infection in the Caribbean in the 1980s (8–11). Bisexuality may play a role by linking different populations during HIV and syphilis epidemics (8,11,12).
Although our study was relatively small and retrospective, it offered a unique opportunity to observe an emerging outbreak in a relatively isolated population of 400,000 in a small geographic area. Early detection also provides a unique opportunity for therapeutic and preventive intervention.
Interpretation of syphilis test results is often difficult in asymptomatic patients, especially when new-generation tests are used in low-prevalence countries (13). In the Caribbean, although yaws was largely eradicated in the 1960s, small outbreaks continue to be reported, as in Martinique from 1974 through 1985. This finding further complicates interpretation of screening results, particularly for older patients.
Syphilis outbreaks will be difficult to detect in regions of the Caribbean where the disease is highly endemic and surveillance is poor. Outbreaks of syphilis and congenital syphilis were reported in Trinidad and Tobago and Jamaica in the 1990s (14,15). Control of outbreaks requires coordinated public health interventions, including new preventive strategies specific for high-risk groups. Preventive messages must be culturally appropriate and must underline the risk for STD transmission by oral sex.
Dr Cabié is a dermatologist and infectious diseases specialist at the Centre Hospitalier Universitaire de Fort-de-France in Martinique. His research interests include the epidemiology of AIDS and sexually transmitted infections.
Acknowledgment
We thank David Young and Chantal Atkinson for editorial assistance.
References
- Centers for Disease Control and Prevention. Transmission of primary and secondary syphilis by oral sex—Chicago, Illinois, 1998–2002. MMWR Morb Mortal Wkly Rep. 2004;53:966–8.PubMedGoogle Scholar
- Williams LA, Klausner JD, Whittington WL, Handsfield HH, Celum C, Holmes KK. Elimination and reintroduction of primary and secondary syphilis. Am J Public Health. 1999;89:1093–7. DOIPubMedGoogle Scholar
- Muller P, Colombani F, Azi M, Belleoud A, Perino C, Chaud P, Syphilis outbreak in Guadeloupe in 2001: link with social precariousness and crack-cocaine addiction [in French]. Bulletin Epidémiologique Hebdomadaire. 2002;48:241–2.
- Fenton KA, Breban R, Vardavas R, Okano T, Martin T, Aral S, Infectious syphilis in high-income settings in the 21st century. Lancet Infect Dis. 2008;8:244–53. DOIPubMedGoogle Scholar
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55:1–94.PubMedGoogle Scholar
- Bouyssou Michel A, Gallay A, Janier M, Dupin N, Halioua B, Alcaraz I, Syphilis surveillance in France, 2000–2006: increase of cases in 2006 [in French]. Bulletin Epidémiologique Hebdomadaire. 2008;5:39–42.
- Taylor MM, Aynalem G, Smith LV, Montoya J, Kerndt P. Methamphetamine use and sexual risk behaviours among men who have sex with men diagnosed with early syphilis in Los Angeles County. Int J STD AIDS. 2007;18:93–7. DOIPubMedGoogle Scholar
- Liautaud B, Pape J, Pamphile M. AIDS in the Caribbean [in French]. Med Mal Infect. 1988;18:687–97. DOIGoogle Scholar
- Bartholomew C, Charles W, Saxinger C, Blattner W, Robert-Guroff M, Raju C, Racial and other characteristics of human T cell leukemia/lymphoma (HTLV-I) and AIDS (HTLV-III) in Trinidad. Br Med J (Clin Res Ed). 1985;290:1243–6. DOIPubMedGoogle Scholar
- Figueroa JP, Brathwaite A, Ward E, DuCasse M, Tscharf I, Nembhard O, The HIV/AIDS epidemic in Jamaica. AIDS. 1995;9:761–8. DOIPubMedGoogle Scholar
- Murphy EL, Gibbs WN, Figueroa JP, Bain B, LaGrenade L, Cranston B, Human immunodeficiency virus and human T-lymphotropic virus type I infection among homosexual men in Kingston, Jamaica. J Acquir Immune Defic Syndr. 1988;1:143–9.PubMedGoogle Scholar
- Cunningham SD, Olthoff G, Burnett P, Rompalo AM, Ellen JM. Evidence of heterosexual bridging among syphilis-positive men who have sex with men. Sex Transm Infect. 2006;82:444–5. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal tests for initial screening—four laboratories, New York City, 2005–2006. MMWR Morb Mortal Wkly Rep. 2008;57:872–5.PubMedGoogle Scholar
- Ali Z. Resurgence of congenital syphilis in Trinidad. J Trop Pediatr. 1990;36:104–8.PubMedGoogle Scholar
- Figueroa JP, Brathwaite AR, Wedderburn M, Ward E, Lweis-Bell K, Amon JJ, Is HIV/STD control in Jamaica making a difference? AIDS. 1998;12(Suppl 2):S89–98.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 16, Number 1—January 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
André Cabié, Service des Maladies Infectieuses et Tropicales, Centre Hospitalier Universitaire de Fort-de-France, 97200 Fort-de-France, Martinique, French West Indies
Top