Volume 16, Number 1—January 2010
Dispatch
Human Listeriosis Caused by Listeria ivanovii
Abstract
Two species of Listeria are pathogenic; L. monocytogenes infects humans and animals, and L. ivanovii has been considered to infect ruminants only. We report L. ivanovii–associated gastroenteritis and bacteremia in a man. This isolate was indistinguishable from prototypic ruminant strains. L. ivanovii is thus an enteric opportunistic human pathogen.
The genus Listeria contains 2 pathogenic species, L. monocytogenes and L. ivanovii (1). They both invade host cells, replicate in the cytosol after phagosomal escape, and spread from cell to cell by polymerizing actin. These mechanisms correlate with the presence in each species of genetic determinants called the inlAB internalization locus, the LIPI-1 intracellular survival pathogenicity island, and the hpt intracellular growth locus (2). However, each species appears to infect different hosts: L. monocytogenes infects humans and ruminants, whereas L. ivanovii is thought to infect ruminants only (2). L. ivanovii have been previously isolated, although rarely, from infected humans, indicating pathogenic potential for humans (Table). We report a case of L. ivanovii infection in a man with a kidney transplant. The ecology of L. ivanovii suggests that the rarity of human listeriosis due to this species reflects not only host tropism factors but also the rare occurrence of this species in the environment, compared with L. monocytogenes.
In January 2007, a 55-year-old man was hospitalized in Paris, France, with a 3-week history of nonbloody diarrhea, vomiting, dehydration, and low-grade fever. Medical history included renal transplantation for chronic renal failure and chronic hepatitis C. Immunosuppressive regimen included mycophenolate mofetil, tacrolimus, and prednisone. At the time of admission, his temperature was 37.8°C and he had moderate and painless abdominal distension. Laboratory values were 5.9 × 109/L leukocytes, 0.4 × 109/L lymphocytes, 9.7 g/dL hemoglobin, 137,000/mL platelets, 470 µmol/L creatinine, and <5 mg/L serum C-reactive protein. Liver tests were within normal limits except γ-glutamyltranferase, which was increased (244 U/L; reference <50 U/L).
Blood cultures yielded coryneform gram-positive rods with intensely β-hemolytic colonies; catalase and esculin hydrolysis test results were positive, consistent with Listeria spp. (1). Because listeriosis was suspected, intravenous amoxicillin and gentamicin therapy was initiated. Cerebrospinal fluid showed no abnormalities by direct examination or culture. Semiquantitative aerobic fecal culture showed the same coryneform gram-positive rods (106 CFU/g). The API Coryne biochemical test (bioMérieux, Marcy l’Étoile, France) identified blood and fecal isolates as Listeria spp. Fecal specimens were negative for Salmonella, Shigella, Yersinia, and Campylobacter spp. After 7 days, intravenous treatment was switched to oral amoxicillin for 2 weeks. The patient’s condition rapidly improved, and control fecal cultures were negative.
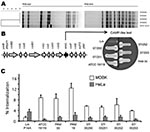
The 3 isolates from blood and 1 from feces were referred to the French National Reference Centre for Listeria (Institut Pasteur, Paris, France). All were identified as L. ivanovii subsp. ivanovii and belonged to L. ivanovii–specific serovar 5. They showed identical profiles by pulsed-field gel electrophoresis (Figure, panel A). Agar diffusion test results were as expected for Listeria spp.: susceptible to amoxicillin and gentamicin; resistant to third-generation cephalosporins, clindamycin, and aztreonam (2). Contrary to L. monocytogenes, which is naturally resistant to fosfomycin in vitro (9), all isolates were susceptible to fosfomycin in vitro, as previously reported (2).
The isolates were compared with prototypic L. ivanovii strains from sheep (American Type Culture Collection 19119 type strain, Ivan Ivanov, 1955, PAM 19, Australia) and goats (PAM 55, Spain). We determined the activation status of the virulence gene regulator PrfA. For L. monocytogenes, the PrfA-regulated factors are mainly expressed in vivo, but for L. ivanovii, they are constitutively overexpressed in vitro (2,11). Some of these virulence factors have easily detectable phenotypes, such as hemolysis on blood agar, PlcB phospholipase activity on egg yolk agar, and Hpt hexose phosphate transporter activity in acidification test (2,12). All isolates were phenotypically identical; they produced broad halos of hemolysis and lecithinase reactions and had positive glucose-1-phosphate acidification test results, reflecting the constitutive activation of the PrfA virulence regulon.
PCR mapping was used to test for L. ivanovii–specific pathogenicity island LIPI-2 (13). LIPI-2 comprises 10 internalin genes and the sphingomyelinase gene smcL and is perfectly conserved within L. ivanovii, including the distantly related subspecies londoniensis (13). All intragenic and intergenic PCRs gave identical results for all strains. The phenotypic marker for LIPI-2, smcL-encoded sphingomyelinase, was assessed by the synergistic hemolysis (CAMP-like) test (13) and was found in all strains (Figure, panel B).
Finally, we performed invasion assays with Madin-Darby bovine kidney (MDBK) cells and HeLa cells (human). Confirming previous observations (13), all L. ivanovii strains were hyperinvasive in MDBK cells and less invasive in HeLa cells compared with L. monocytogenes (Figure, panel C). Invasion assays expressing human E-cadherin or not did not show substantial differences, suggesting that L. ivanovii InlA does not interact with E-cadherin, in contrast to L. monocytogenes InlA (6) (data not shown). The 4 patient isolates showed slightly lower invasion capacity in MDBK cells than did isolates from ruminants but were still hyperinvasive relative to L. monocytogenes.
We found 3 other well-documented cases of L. ivanovii–associated human infection (Table) 1 febrile diarrhea (7) and 2 bacteremia cases (8,10). The infections were associated with AIDS, metastatic carcinoma, or substance abuse; 2 patients were >60 years of age. Thus, as for L. monocytogenes (1), human L. ivanovii infection is associated with immunodeficiency, underlying debilitating conditions, or advanced age. In at least 3 other instances, bacteria were found in human samples, 2 in fetoplacental tissue and lochia and 1 in a mesenteric lymph node (4,5) (Table). The pathologic changes associated with L. ivanovii in humans appear similar to those in ruminants, i.e., fetoplacental infections and septicemia (often accompanied by enteritis). Typically, meningoencephalitis is not caused by L. ivanovii in ruminants, whereas it is a hallmark of L. monocytogenes infection in ruminants and humans (1). Lack of central nervous system involvement could be a general characteristic of L. ivanovii infection regardless of host species. The specific pathogenic features of L. ivanovii may be caused by sequence differences in virulence genes shared with L. monocytogenes or by differences in the gene content of these 2 species (1,6).
These human cases raise questions about the supposed specificity of L. ivanovii for ruminants. Although the rare occurrence of L. ivanovii infections in humans (3) could result from lower pathogenicity for humans, it may reflect ecologic characteristics of the species. L. ivanovii is isolated only occasionally from animals or environmental sources (2,4,5), suggesting a limited distribution in nature, including in food. Therefore, the few human cases of L. ivanovii infection reported might correspond to what would be proportionally expected for a species with such sporadic occurrence.
That gastroenteritis preceded bacteremia and that the same isolates were found in the feces strongly indicate a foodborne infection in the patient reported here and that L. ivanovii causes gastroenteritis in humans, as reported for L. monocytogenes (14). Days before onset of gastroenteritis, the patient had eaten artisanal goat cheese made from raw milk. Unfortunately, no cheese sample was available for bacteriologic investigation. Although the portal of entry of L. ivanovii has not been formally established, L. ivanovii infection in ruminants is associated with eating spoiled silage or hay, as happens with L. monocytogenes, suggesting foodborne origin. L. ivanovii has been isolated from food, including goat milk (15).
Simultaneous detection of L. ivanovii in the feces and blood of a human, together with previous association between L. ivanovii and human mesenteric adenitis (5), suggests that these bacteria can cross the intestinal barrier in humans, cause gastroenteritis, and disseminate into the bloodstream. Although L. monocytogenes are by far the leading cause of human listeriosis, our report shows that L. ivanovii can also cause bacteremia in immunocompromised, debilitated patients.
Dr Guillet is a clinical microbiologist in the Necker Hospital, Paris Descartes University, in Paris. She has particular interests in bacterial infections in immunodeficient patients and in pediatric infectious diseases.
Acknowledgments
We thank Véronique Goulet for helpful discussions.
M.L. received financial support from Assistance Publique–Hôpitaux de Paris, Université Paris Descartes, Institut Pasteur, Institut de Veille Sanitaire, and Institut National de la Santé et de la Recherche Médicale. J.V.-B. received Wellcome Trust program grant 074020.
References
- Seeliger HPR, Jones D. Genus Listeria. In: Sneath PHA, Mair NS, Sharpe ME, and Holt JG, editors. Bergey’s manual of systematic bacteriology, Vol. 2. Baltimore: Williams & Wilkins; 1986. p. 1235–45.
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. DOIPubMedGoogle Scholar
- Busch LA. Human listeriosis in the United States, 1967–1969. J Infect Dis. 1971;123:328–32.PubMedGoogle Scholar
- Rocourt J, Seeliger HP. Distribution des espèces du genre Listeria. Zentralbl Bakteriol Mikrobiol Hyg [A]. 1985;259:317–30.PubMedGoogle Scholar
- Elischerova K, Cupkova E, Urgeova E, Lysy J, Sesevickova A. Isolation of Listeria ivanovii in Slovakia [in Slovak]. Cesk Epidemiol Mikrobiol Imunol. 1990;39:228–36.PubMedGoogle Scholar
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–63. DOIPubMedGoogle Scholar
- Cummins AJ, Fielding AK, McLauchlin J. Listeria ivanovii infection in a patient with AIDS. J Infect. 1994;28:89–91. DOIPubMedGoogle Scholar
- Snapir YM, Vaisbein E, Nassar F. Low virulence but potentially fatal outcome—Listeria ivanovii. Eur J Intern Med. 2006;17:286–7. DOIPubMedGoogle Scholar
- Troxler R, von Graevenitz A, Funke G, Wiedemann B, Stock I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin Microbiol Infect. 2000;6:525–35. DOIPubMedGoogle Scholar
- Lessing MP, Curtis GD, Bowler IC. Listeria ivanovii infection. J Infect. 1994;29:230–1. DOIPubMedGoogle Scholar
- Scortti M, Lacharme-Lora L, Wagner M, Chico-Calero I, Losito P, Vázquez-Boland JA. Coexpression of virulence and fosfomycin susceptibility in Listeria: molecular basis of an antimicrobial in vitro-in vivo paradox. Nat Med. 2006;12:515–7. DOIPubMedGoogle Scholar
- Ripio MT, Brehm K, Lara M, Suarez M, Vazquez-Boland JA. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179:7174–80.PubMedGoogle Scholar
- Domínguez-Bernal G, Müller-Altrock S, González-Zorn B, Scortti M, Herrmann P, Monzó HJ, A spontaneous genomic deletion in Listeria ivanovii identifies LIPI-2, a species-specific pathogenicity island encoding sphingomyelinase and numerous internalins. Mol Microbiol. 2006;59:415–32. DOIPubMedGoogle Scholar
- Dalton CB, Austin CC, Sobel J, Hayes PS, Bibb WF, Graves LM, An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–5. DOIPubMedGoogle Scholar
- Gaya P, Saralegui C, Medina M, Nuñez M. Occurrence of Listeria monocytogenes and other Listeria spp. in raw caprine milk. J Dairy Sci. 1996;79:1936–41.PubMedGoogle Scholar
Figure
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 16, Number 1—January 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondance: Marc Lecuit, Centre d’Infectiologie Necker-Pasteur, Service des Maladies Infectieuses et Tropicales, Hôpital Necker–Enfants Malades, 149 rue de Sèvres, 75015 Paris, France
Top