Volume 16, Number 12—December 2010
Research
Eastern Equine Encephalitis Virus in Mosquitoes and Their Role as Bridge Vectors
Cite This Article
Citation for Media
Abstract
Eastern equine encephalitis virus (EEEV) is maintained in an enzootic cycle involving Culiseta melanura mosquitoes and avian hosts. Other mosquito species that feed opportunistically on mammals have been incriminated as bridge vectors to humans and horses. To evaluate the capacity of these mosquitoes to acquire, replicate, and potentially transmit EEEV, we estimated the infection prevalence and virus titers in mosquitoes collected in Connecticut, USA, by cell culture, plaque titration, and quantitative reverse transcription–PCR. Cs. melanura mosquitoes were the predominant source of EEEV (83 [68%] of 122 virus isolations) and the only species to support consistently high virus titers required for efficient transmission. Our findings suggest that Cs. melanura mosquitoes are primary enzootic and epidemic vectors of EEEV in this region, which may explain the relative paucity of human cases. This study emphasizes the need for evaluating virus titers from field-collected mosquitoes to help assess their role as vectors.
During the past 6 years, eastern equine encephalitis virus (EEEV; family Togaviridae, genus Alphavirus) has reemerged in the northeastern United States and resulted in 26 human cases of infection and 9 deaths (ArboNET; Centers for Disease Control and Prevention, Atlanta, GA, USA). Virus transmission has intensified throughout this region, spread to locales where it had not been previously detected, and extended north into New Hampshire and Maine, USA, and Nova Scotia, Canada (1). Disease outbreaks caused by EEEV occur at irregular intervals when underlying ecologic conditions favor virus amplification and overflow into human and equine populations.
EEEV is perpetuated in an enzootic cycle involving ornithophilic mosquitoes (primarily Culiseta melanura) and passerine birds in freshwater swamps (2,3). Human and equine cases occur infrequently despite relatively high rates of EEEV infection in Cs. melanura during virus amplification (ArboNET). Other mosquito species such as Aedes vexans, Coquillettidia perturbans, Ochlerotatus canadensis, and Oc. sollicitans have been implicated as epidemic/epizootic bridge vectors from viremic birds to horses and humans (4–6). These species are competent vectors of EEEV (7–9) and may acquire virus infection during disease outbreaks by feeding occasionally on birds but prefer mammalian hosts (10–14). Although Cs. melanura mosquitoes feed infrequently on mammals (10,12,15), their ability to serve as a bridge vector may be offset by a much higher prevalence of EEEV infection in this species.
One criterion used for incriminating enzootic and bridge vectors is based on the frequency of virus detection from each candidate species (16). Typically, mosquitoes are collected from disease-endemic sites, sorted into pools by trap location and species, and screened for virus by cell culture or molecular methods. This procedure provides critical information on the identity, spatial and temporal distribution, and proportion of virus-infected mosquitoes and forms the basis for many arbovirus surveillance programs. Nevertheless, virus titers may vary considerably within infected mosquitoes (17) and reflect the duration of the extrinsic incubation period and the ability of these mosquitoes to support virus replication, which is a necessary precondition for mosquitoes becoming infectious (18). The virus must undergo several rounds of replication in the mosquito midgut and salivary glands before being biologically transmitted to the vertebrate host when the mosquito salivates during blood feeding.
In Connecticut in 2009, EEEV activity increased substantially, and we isolated numerous viruses from Cs. melanura mosquitoes and potential bridge vectors. To evaluate the capacity of these mosquitoes to replicate and potentially transmit virus, we estimated EEEV titers from virus-positive mosquito pools with the expectation that the most efficient vectors will support consistently high virus titers.
Mosquito Collections
Mosquitoes were collected at 91 trapping locations statewide as a part of the Connecticut Mosquito and Arbovirus Surveillance Program during June–October 2009. Each trapping site was sampled on average weekly and at least every 10 days by means of CO2-baited Centers for Disease Control light traps and gravid traps that were operated overnight. Adult mosquitoes were transported back to the laboratory alive and sorted on chill tables by trap location and according to species by using taxonomic keys (19). Mosquitoes containing visible blood in the abdomen were removed and not included in this study. Mosquitoes were combined into pools of <50 individuals, placed in microcentrifuge tubes containing a copper BB, and stored at –70°C until virus testing.
Virus Isolation and Identification
Mosquitoes were homogenized in 1.0–1.5 mL of phosphate-buffered saline supplemented with 30% heat-inactivated rabbit serum, 0.5% gelatin, and antibacterial and antifungal drugs by using a vibration mill set for 4 min at 25 cycles/s. Homogenates were centrifuged at 4°C for 7 min at 520 × g, and 100 µL of the supernatant was placed on confluent Vero cells growing in 25-cm2 flasks containing minimal essential media, 5% fetal bovine serum, and antibacterial and antifungal drugs. Cell cultures were maintained at 37°C in an atmosphere of 5% CO2 and monitored daily for cytopathic effect during days 3–7 postinfection. Infected cell supernatants were harvested and stored at –80°C.
Mosquito pools that yielded infectious virus in cell culture were directly tested for EEEV by quantitative reverse transcription–PCR (qRT-PCR). RNA was extracted from mosquito pool homogenates by using spin columns and reagents in a viral RNA kit (QIAGEN, Valencia, CA, USA). A total of 2.5 µL of this preparation was added to a 25-µL qRT-PCR by using the TaqMan One-Step RT-PCR Kit (Applied Biosystems, Foster City, CA, USA) and primers/probe (9391/9459c/9414probe) (20). Amplification was performed as follows: 1 cycle at 50°C for 20 min and 95°C for 10 min and 50 cycles at 95°C for 15 s and 60°C for 1 min. Positive results were based on cycle threshold (Ct) values when the change in fluorescence increased above the baseline threshold value calculated by using IQ5 Optical System Software (Bio-Rad, Hercules, CA, USA). Samples that failed to amplify or yielded Ct values >30 were reconfirmed by reisolation of EEEV in cell culture. Supernatants from virus cultures were also tested for EEEV by qRT-PCR or by conventional RT-PCR and by sequencing a portion of the nonstructural protein gene of EEEV as described (21).
Plaque Titration
Plaque titrations were performed on confluent Vero cell cultures growing in 12-well plates. Ten-fold dilutions of mosquito pool homogenates were placed in triplicate onto cell monolayers and absorbed for 1 h at 37°C in an atmosphere of 5% CO2. Cells were overlaid with 1% methylcellulose in minimal essential medium, 5% fetal bovine serum, and antimicrobial and antifungal drugs and returned to the incubator. After 3 days, cells were fixed overnight in 7.4% formaldehyde and stained with 1% crystal violet so plaques could be visualized.
Statistical Analyses
The Pooled Infection Rate add-in for Excel (Microsoft, Redmond, WA, USA) was used to calculate virus infection rates (per 1,000 mosquitoes) and 95% confidence intervals (CIs) on the basis of maximum likelihood estimation (MLE) (22). All other statistical tests were performed by using Instat version 3.06 (GraphPad Software, San Diego, CA, USA). The Mann-Whitney U test was used for comparing median virus titers in pools for Cs. melanura mosquitoes and 6 other species from which a minimum of 3 positive pools were obtained (Ae. cinereus, Anopheles punctipennis, Culex salinarius, Oc. canadensis, and Uranotaenia sapphirina mosquitoes). The relationship between Ct values and log10 virus titers were analyzed by regression analysis. Statistical significance was assigned at p<0.05 or by nonoverlapping 95% confidence intervals.
In 2009, a total of 291,641 mosquitoes (35 species) were collected and processed as 16,909 pools for virus isolation. EEEV was isolated from 122 mosquito pools, which represented 14 species and 7 genera (Table), obtained during August 17–October 27 in 25 of 91 trapping locations. Cs. melanura mosquitoes yielded the greatest number of EEEV isolations (n = 83) and was followed by Oc. canadensis (n = 10) and Ae. cinereus (n = 6) mosquitoes. Relatively few (<4) or no EEEV isolates were obtained from the remaining mosquito species. EEEV infection rates were higher in Cs. melanura mosquitoes (MLE 3.44, 95% CI 2.76–4.24) than in all other mosquito species tested except An. quadrimaculatus (MLE 3.27, 95% CI 0.59–10.55) and Ur. sapphirina (MLE 1.35, 95% CI 0.44–3.23) mosquitoes.
Mosquito pools that yielded EEEV in cell culture were directly tested by qRT-PCR. A total of 108 mosquito pools had positive Ct values (<37) on the basis of criteria established by Lambert et al. (20), 7 mosquito pools were equivocal (Ct 37.6–39.0), and 7 isolates failed to amplify after 50 amplification cycles. All mosquito pools that were negative or had Ct values >30 by qRT-PCR were retested in Vero cell culture and confirmed by reisolation of EEEV. Mean Ct values were lowest for Cs. melanura mosquitoes (Ct 22.3) and exceeded 30 for all other mosquito species (Table), which suggested species-specific differences in virus titer.
Concentration of infectious virus was estimated from positive mosquito pools by plaque titration in Vero cell culture. Cs. melanura mosquitoes had significantly higher virus titers (mean 6.55 log10 PFU/mosquito pool) than all other mosquito species for which statistical comparisons were possible (p<0.01 by Mann-Whitney U test). Ae. cinereus and Oc. canadensis had the next highest virus titers (2.92 log10 PFU/mosquito pool and 2.82 log10 PFU/mosquito pool, respectively), and mean titers ranged from <0.8 log10 PFU/mosquito pool to 1.69 log10 PFU/mosquito pool in the remaining species. The percentage of Cs. melanura mosquito pools with high virus titers (>3.0 log10 PFU/mosquito pool) was 88% compared with 0%–16.7% for the 13 other mosquito species (Table).
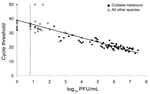
The relationship between Ct values and PFU estimated from EEEV-infected mosquito pools is shown in the Figure. A strong negative correlation was found between Ct values and PFU/mL in positive mosquito pools by regression analysis (slope –3.1, y-intercept 39; p>0.0001), which indicated a predictive relationship between these 2 measures of virus concentration.
Our analysis of EEEV-positive mosquito pools showed major differences in virus titer among different mosquito species obtained in Connecticut. Cs. melanura was the only species in which titers developed that were associated with EEEV transmission, estimated to be 4–7 logs of virus in virus-transmitting mosquitoes in previous studies (23–25). This finding suggests that EEEV is transmitted primarily by Cs. melanura mosquitoes in this region of the northeastern United States, despite repeated virus isolations from other mosquito species.
Infrequent human and horse infections by EEEV may arise when Cs. melanura mosquitoes occasionally feed on mammals rather than by participation of another epidemic/epizootic bridge vector. Prior studies identified mammalian-derived blood meals in 1%–10% of Cs. melanura mosquitoes obtained in Massachusetts, Connecticut, and New York (10,12,15) and provided a direct conduit for virus transmission by this species to horses and humans. These analyses, in conjunction with observations on vector longevity (26), vector competence (8,25,27), and prevalence of EEEV infection in Cs. melanura mosquitoes (ArboNET), suggest that this species could serve as an enzootic and epidemic/epizootic bridge vector of EEEV. We provide additional support for this hypothesis by estimating virus titers in field-collected mosquitoes, which enabled us to determine which infected mosquitoes could potentially transmit virus.
In this study and previous studies, most EEEV isolations have been from either Cs. melanura or Cs. morsitans mosquitoes, depending on the region. These 2 species comprised 46%–100% of all isolations from field-collected mosquitoes in published studies (1,28–35) and 62%–92% of all EEEV-positive mosquito pools reported to the Centers for Disease Control and Prevention through ArboNET during 2004–2009. The remaining virus isolations come from a diversity of species, some of which were implicated as bridge vectors largely on the basis of local abundance, temporal and spatial distribution in relationship to human cases, and virus isolation during epidemics.
Ae. vexans mosquitoes are often mentioned as a possible bridge vector of EEEV in the northeastern United States (1,3,5,12,33). Their distribution and late season abundance overlap with distribution of human cases, and they will feed opportunistically on avian and mammalian hosts. However, vector competence trials have ranked this species as an inefficient vector in the laboratory (9); it failed to transmit virus in 1 study (8). Our results suggest a negligible role for this species. EEEV was isolated only twice from Ae. vexans mosquitoes, and the 2 positive pools showed low virus titers, which reinforced previous findings. A study by Nasci and Mitchell (17) reported low EEEV titers (<3.0 log10 PFU/mL) in all Ae. vexans mosquito pools tested (n = 4).
Oc. canadensis mosquitoes were the second major source of EEEV after Cs. melanura mosquitoes and accounted for 10 (8%) of 122 virus isolations in this study. EEEV has been detected in this species throughout the northeastern United States, including New Jersey, Rhode Island, New York, Massachusetts, and New Hampshire (33,34,36) (ArboNET) and is a moderately competent vector in the laboratory (8). Oc. canadensis is the most frequently trapped mosquito in Connecticut and is found in a variety of habitats that include freshwater swamps in which EEEV is found. Adult populations peak in late June–early July but extend into fall (19), particularly if a second hatch occurs during periods of heavy rainfall. Host-seeking females feed mainly on mammals, including horses and humans and occasionally birds (11,14). This finding suggests that Oc. canadensis mosquitoes may be a bridge vector in Connecticut, but its relative contribution appears to be minor when virus titers in field-collected mosquito pools are considered. We detected high virus titers in only 1 pool of Oc. canadensis mosquitoes (3.2 log10 PFU/mL), which is consistent with observations in which 0 of 2 EEEV-positive pools of Oc. canadensis mosquitoes contained high titers of virus (>3.0 log10 PFU/mL) (17).
EEEV was also isolated from 6 Ae. cinereus mosquitoes, which represented the third most common source of virus. Of these pools, 1 contained high titers of virus (3.5 log10 PFU/mL). This species may serve as a potential bridge vector on the basis of certain ecologic and behavioral criteria. Host-seeking females are abundant during June–October in many habitats throughout Connecticut (19). This species feeds opportunistically on mammals and birds but prefers mammals (11). The ability of this species to transmit EEEV has not been evaluated in the laboratory. Therefore, its contribution as a vector requires further evaluation.
We did not isolate EEEV from Oc. sollicitans or Cq. perturbans, 2 mosquito species implicated as likely bridge vectors in other epidemiologic settings (4,5,34,37). The eastern salt marsh mosquito, Oc. sollicitans, is an aggressive biter of humans that may transmit virus in the mid-Atlantic region but its coastal distribution does not overlap with that of human and equine cases in New England. Cq. perturbans mosquitoes are commonly trapped in Connecticut (35,389 females collected in 2009) and are found near EEEV foci. Host-seeking females emerge as 1 generation that peaks in early July and then decrease sharply by mid-August when EEEV begins to amplify in Connecticut (19). EEEV has been isolated from Cq. perturbans mosquitoes throughout the eastern United States, including Connecticut in other years (6,28,33,37) (ArboNET). In the study by Nasci and Mitchell (17), 13 (65%) of 20 pools of Cq. perturbans mosquitoes contained high titers of EEEV (>3.0 log10 PFU/mL), which suggested a bridge role for this species. However, its low abundance in late August and September when EEE activity is greatest argues against its involvement as a primary epidemic vector in this region.
Our results obtained by qRT-PCR corresponded to those estimated by plaque titration, which provided a basis for interpreting Ct values estimated directly from mosquito pools. On the basis of the fitted line estimated by regression analysis in the Figure, a Ct<29.8 corresponded to virus titers >3.0 log10 PFU/mL. We used this titer as a threshold for classifying low or high virus titers in mosquito pools for purposes of comparison and to discern whether a virus infection was established and replication occurred in the mosquito vector. Competent vectors must support virus replication during the extrinsic incubation period to be able to transmit virus. However, quantitative data for the minimum virus titer necessary for transmission are limited or absent for most virus–vector systems. Despite these uncertainties, investigations showed that mosquitoes transmitted virus when their body titers exceeded 4–5 logs of virus for EEEV (23–25) and western equine encephalitis virus (38,39). Virus transmission was usually associated with mosquitoes containing >5 logs of virus. However, some females with high virus titers failed to transmit virus in these studies. On the basis of these considerations, we believe that mosquito pools exhibiting low virus titers (<3.0 log10 PFU/mosquito pool) would be highly unlikely to contain mosquitoes capable of transmitting virus at the time of sampling, whereas detection of high virus titers does not necessarily predict that infected mosquitoes are capable of virus transmission. The strength of the data in our study is based on consistent detection of high virus titers from only 1 competent mosquito vector (Cs. melanura).
Most of the pools that showed EEEV in cell culture also showed positive results by qRT-PCR (Ct <37). However, our ability to reisolate EEEV from another 14 mosquito pools with either equivocal or negative results indicates that Vero cell culture is a highly sensitive assay for detection of EEEV. Mosquito pools containing infectious virus should be reconfirmed and quantified by another independent method; qRT-PCR is currently the most convenient method to accomplish this test.
Published data on virus titers from field-collected mosquitoes are currently limited to 1 study by Nasci and Mitchell (17), and their findings were consistent with our observations in Cs. melanura, Ae. vexans, and Oc. canadensis mosquitoes. Virus titer variation observed among mosquito species could reflect differences in vector competence. EEEV was shown to infect, replicate, and disseminate rapidly in Cs. melanura mosquitoes and was detected in the salivary glands 2–3 days after infection (25). Moreover, a larger proportion of Cs. melanura mosquitoes transmitted EEEV than Aedes, Anopheles, Coquillettidia, Culex, and Ochlerotatus mosquitoes in a direct laboratory comparison (8). Virus titers will also vary according to the duration of the extrinsic incubation period. One limitation of our study is that because we did not know the age structure of the mosquito population, we could not control this variable.
Low virus titers may have also been caused by contamination of infected mosquito fragments during sorting and testing procedures. Although we cannot preclude this possibility, we made concerted efforts to ensure accurate testing. Mosquitoes were sorted into mosquito pools on chill tables, and mosquito pools were processed for virus isolation in a separate facility according to standard practices. EEEV-positive pools were directly tested and quantified by using 2 independent tests (plaque titration and qRT-PCR). Our results were consistent with those of other studies (1,28–35), which showed that most EEEV isolations were from Cs. melanura mosquitoes and several other mosquito species.
Our findings highlight the need to consider virus titer when interpreting virus isolation or PCR detection data for field-collected mosquitoes. Although we isolated EEEV from several mosquito species, Cs. melanura was the only species that had consistently high titers of EEEV sufficient for transmission. This finding may help reconcile the paucity of symptomatic human and equine cases, despite frequent detection or isolation of virus from mammalophilic mosquitoes during episodes of virus amplification. These results should be verified in other regions, where involvement of other locally abundant mosquitoes is suspected during disease outbreaks. When information on virus titers in mosquitoes is considered, the number of candidate vectors may be reduced to a few key species that are capable of supporting virus transmission in nature.
Dr Armstrong is a virologist at The Connecticut Agricultural Experiment Station, Center for Vector Biology and Zoonotic Diseases. His research interests are the ecology and molecular epidemiology of mosquito-borne viruses.
Dr Andreadis is chief medical entomologist in the Department of Environmental Sciences and Center for Vector Biology and Zoonotic Diseases at the Connecticut Agricultural Experiment Station. His research interests are the invasion biology of exotic mosquitoes, epidemiology of vector-borne diseases, and biological control of mosquitoes.
Acknowledgments
We thank Shannon Finan, John Shepard, Michael Thomas, and Brittany Crowley for technical assistance.
This study was supported in part by grants from the Centers for Disease Control and Prevention (U50/CCU116806-01-1) and the US Department of Agriculture (58-6615-1-218, CONH00768, and CONH00773).
References
- Centers for Disease Control and Prevention. Eastern equine encephalitis—New Hampshire and Massachusetts, August–September 2005. MMWR Morb Mortal Wkly Rep. 2006;55:697–700.PubMedGoogle Scholar
- Scott TW, Weaver SC. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989;37:277–328. DOIPubMedGoogle Scholar
- Komar N, Spielman A. Emergence of eastern encephalitis in Massachusetts. Ann N Y Acad Sci. 1994;740:157–68. DOIPubMedGoogle Scholar
- Crans WJ. The status of Aedes sollicitans as an epidemic vector of eastern equine encephalitis virus in New Jersey. Mosq News. 1977;37:85–9.
- Hayes RO, Beadle LD, Hess AD, Sussman O, Bonese MJ. Entomological aspects of the 1959 outbreak of eastern encephalitis in New Jersey. Am J Trop Med Hyg. 1962;11:115–21.PubMedGoogle Scholar
- Howard JJ, Morris CD, Emord DE, Grayson MA. Epizootiology of eastern equine encephalitis virus in upstate New York, USA. VII. Virus surveillance 1978–85, description of 1983 outbreak, and series conclusions. J Med Entomol. 1988;25:501–14.PubMedGoogle Scholar
- Davis WA. A study of birds and mosquitoes as hosts for the virus of eastern equine encephalomyelitis. Am J Hyg. 1940;32:45–59.
- Vaidyanathan R, Edman JD, Cooper LA, Scott TW. Vector competence of mosquitoes (Diptera:Culicidae) from Massachusetts for a sympatric isolate of eastern equine encephalomyelitis virus. J Med Entomol. 1997;34:346–52.PubMedGoogle Scholar
- Chamberlain RW, Sikes RK, Nelson DB, Sudia WD. Studies on the North American arthropod-borne encephalitides. VI. Quantitative determinations of virus-vector relationships. Am J Hyg. 1954;60:278–85.PubMedGoogle Scholar
- Molaei G, Andreadis TG. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J Med Entomol. 2006;43:1088–93. DOIPubMedGoogle Scholar
- Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, U.S.A.: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol. 2008;45:1143–51. DOIPubMedGoogle Scholar
- Nasci RS, Edman JD. Blood-feeding patterns of Culiseta melanura (Diptera: Culicidae) and associated sylvan mosquitoes in southeastern Massachusetts eastern equine encephalitis enzootic foci. J Med Entomol. 1981;18:493–500.
- Edman JD. Host-feeding patterns of Florida mosquitoes I. Aedes, Anopheles, Coquillettidia, Mansonia, and Psorophora. J Med Entomol. 1971;8:687–95.PubMedGoogle Scholar
- Magnarelli LA. Host feeding patterns of Connecticut mosquitoes (Diptera: Culicidae). Am J Trop Med Hyg. 1977;26:547–52.PubMedGoogle Scholar
- Molaei G, Oliver J, Andreadis TG, Armstrong PM, Howard JJ. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am J Trop Med Hyg. 2006;75:1140–7.PubMedGoogle Scholar
- Eldridge BF. The epidemiology of arthropod-borne diseases. In: Eldridge BF, Edman JD, editors. Medical entomology: a textbook on public health and veterinary problems caused by arthropods. Dordrecht (the Netherlands): Kluwer Academic Publishers; 2004. p. 165–85.
- Nasci RS, Mitchell CJ. Arbovirus titer variation in field-collected mosquitoes. J Am Mosq Control Assoc. 1996;12:167–71.PubMedGoogle Scholar
- Hardy JL. Susceptibility and resistance of vector mosquitoes. In: Monath TP, editor. The arboviruses: epidemiology and ecology. Boca Raton (FL): CRC Press; 1988. p. 87–126.
- Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. New Haven (CT): The Connecticut Agricultural Experiment Station; 2005.
- Lambert AJ, Martin DA, Lanciotti RS. Detection of North American eastern and western equine encephalitis viruses by nucleic acid amplification assays. J Clin Microbiol. 2003;41:379–85. DOIPubMedGoogle Scholar
- Armstrong PM, Andreadis TG, Anderson JF, Stull JW, Mores CN. Tracking eastern equine encephalitis virus perpetuation in the northeastern United States by phylogenetic analysis. Am J Trop Med Hyg. 2008;79:291–6.PubMedGoogle Scholar
- Biggerstaff BJ. PooledInfRate, version 3.0: a Microsoft Excel add-in to compute prevalence estimates from pooled samples. Fort Collins (CO): Centers for Disease Control and Prevention; 2006.
- Collins WE, Harrison AJ, Jumper JR. Infection and transmission studies with eastern encephalitis virus in Anopheles albimanus and Anopheles quadrimaculatus. Mosq News. 1965;25:296–300.
- Collins WE, Harrison AJ, Jumper JR. Infection and transmission thresholds of eastern encephalitis virus in Aedes aegypti as determined by a membrane feeding technique. Mosq News. 1965;25:293–5.
- Weaver SC, Scott TW, Lorenz LH. Patterns of eastern equine encephalomyelitis virus infection in Culiseta melanura (Diptera: Culicidae). J Med Entomol. 1990;27:878–91.PubMedGoogle Scholar
- Morris CD. Phenology of trophic amd gonobiologic states in Culiseta morsitans and Culiseta melanura (Diptera: Culicidae). J Med Entomol. 1984;21:38–51.PubMedGoogle Scholar
- Howard JJ, Wallis RC. Infection and transmission of eastern equine encephalomyelitis virus with colonized Culiseta melanura (Coquillett). Am J Trop Med Hyg. 1974;23:522–5.PubMedGoogle Scholar
- Andreadis TG, Anderson JF, Tirrell-Peck SJ. Multiple isolations of eastern equine encephalitis and highlands J viruses from mosquitoes (Diptera: Culicidae) during a 1996 epizootic in southeastern Connecticut. J Med Entomol. 1998;35:296–302.PubMedGoogle Scholar
- Chamberlain RW, Sudia WD, Coleman PH, Johnston JG Jr, Work TH. Arbovirus isolations from mosquitoes collected in Waycross, Georgia, 1963, during an outbreak of equine encephalitis. Am J Epidemiol. 1969;89:82–8.PubMedGoogle Scholar
- Sudia WD, Chamberlain RW, Coleman PH. Arbovirus isolations from mosquitoes collected in south Alabama, 1959–1963, and serologic evidence of human infection. Am J Epidemiol. 1968;87:112–6.PubMedGoogle Scholar
- Williams JE, Watts DM, Young OP, Reed TJ. Transmission of eastern (EEE) and western (WEE) encephalitis to bobwhite sentinels in relation to density of Culiseta melanura mosquitoes. Mosq News. 1972;32:188–92.
- Bigler WJ, Lassing EB, Buff EE, Prather EC, Beck EC, Hoff GL. Endemic eastern equine encephalomyelitis in Florida: a twenty-year analysis, 1955–1974. Am J Trop Med Hyg. 1976;25:884–90.PubMedGoogle Scholar
- Edman JD, Timperi R, Werner B. Epidemiology of eastern equine encephalitis in Massachusetts. Journal of the Florida Mosquito Control Association. 1993;64:84–96.
- Howard JJ, Grayson MA, White DJ, Morris CD. Eastern equine encephalitis in New York State. Journal of the Florida Mosquito Control Association. 1994;65:1–7.
- Wellings FM, Lewis AL, Pierce LV. Agents encountered during arboviral ecological studies: Tampa Bay area, Florida, 1963–1970. Am J Trop Med Hyg. 1972;21:201–13.PubMedGoogle Scholar
- Gettman AD. Epidemiology of eastern equine encephalitis in Rhode Island. Journal of the Florida Mosquito Control Association. 1993;64:104–5.
- Nasci RS, Berry RL, Restifo RA, Parsons MA, Smith GC, Martin DA. Eastern equine encephalitis virus in Ohio during 1991. J Med Entomol. 1993;30:217–22.PubMedGoogle Scholar
- Kramer LD, Hardy JL, Presser SB. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine encephalomyelitis virus. Am J Trop Med Hyg. 1983;32:1130–9.PubMedGoogle Scholar
- Reisen WK, Meyer RP, Presser SB, Hardy JL. Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera: Culicidae). J Med Entomol. 1993;30:151–60.PubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 16, Number 12—December 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Philip M. Armstrong, Center for Vector Biology and Zoonotic Diseases, Department of Environmental Sciences, The Connecticut Agricultural Experiment Station, 123 Huntington St, PO Box 1106, New Haven, CT 06504, USA
Top