Volume 16, Number 9—September 2010
Letter
Invasive Klebsiella pneumoniae Infections, California, USA
Cite This Article
Citation for Media
To the Editor: A distinctive form of tissue-invasive community-associated Klebsiella pneumoniae infection, typified by primary liver abscess and bacteremia, has been well known in Asia for 2 decades (1–4). Association of these infections with a hypermucoviscous phenotype was discovered in 2004 (5). Certain genetic and virulence features were elucidated in that and subsequent reports (6).
The phenotype, easily detected at the bench by the string test (5), has been associated with a chromosomal gene, the mucoviscosity-associated gene A (magA), and a plasmid gene, the regulator of the mucoid phenotype A gene (rmpA). Usually serotypes K1 and K2 can be demonstrated. Hypermucoviscous isolates demonstrate increased virulence in mice, are serum insensitive, and resist phagocytosis (5).
Reports of such infections from Europe and North America are rare. Recently 2 of us (L.L and B.F.) reported 4 cases in persons seeking care at the Alameda County Medical Center in Oakland, California, USA (7). We report 9 more cases, 7 from Alameda County Medical Center and 2 from St. Rose Hospital in Alameda County. The 13 cases are described in aggregate.
One case occurred in 2006, 3 in 2007, 7 in 2008, and 2 in January 2009. Median patient age was 52 years (range 37–70 years), and 9 were men. Ten patients were born in Asia (Philippines, Vietnam, South Korea, Cambodia, and Yemen), but all had emigrated years earlier. Two patients were born in the United States (1 Filipino and 1 African American); the birth site of 1 Filipino was unknown. Five patients had no underlying illness. Seven had diabetes mellitus, 1 had α-thalassemia, 2 had uncontrolled cancer, and 1 had preexisting multiple organ failure. Three patients had gallstones.
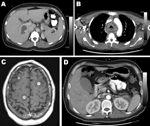
Case-patients exhibited diverse clinical features. The site of infection was easily detected by dramatically abnormal results of computed tomographic scan or magnetic resonance imaging (Figure). Seven patients had liver abscesses. One of these patients also had cholecystitis and choledocholithiasis. One other patient with healthcare-associated bacteremia most likely had multiple small liver abscesses that were superinfected cancer metastases.
Two patients had neck abscesses, 1 complicated by extensive descending mediastinitis (Figure). One patient had kidney abscesses complicated by septic and bland pulmonary emboli and numerous brain abscesses detected by magnetic resonance imaging (Figure).
Healthcare-associated bacteremia occurred in 3 patients. One patient had sustained bacteremia without a clear source on hospital day 115. Two patients with uncontrolled cancer also had healthcare-associated bacteremia.
Venous thrombotic complications occurred in 6 patients, most diagnosed at admission. Two patients had bland pulmonary emboli, and 1 patient with uncontrolled cancer had thrombosis of an upper extremity vein and both femoral veins. Two other patients had septic pulmonary emboli suggested by computed tomographic scan. One patient with α-thalassemia had kidney abscesses and renal vein thrombosis, followed by femoral deep vein thrombosis and pulmonary embolus. A patient with a neck abscess had a thrombosed neck vein at surgery.
Four patients died, and 1 was lost to follow-up, for a death rate of at least 31%. None died directly of sepsis.
All isolates were resistant in vitro to ampicillin but susceptible to all other antimicrobial drugs tested. Genotyping was performed on isolates from 4 patients. One isolate contained the rmpA gene; 3 contained rmpA and magA genes. Three of these isolates also underwent capsule serotyping; 2 were type K1 and 1 was K2.
We found 4 additional patients infected with K. pneumoniae in 2009 who did not have invasive infections. Briefly, a 21-year-old pregnant recent emigrant from Yemen and a 35-year-old Hispanic pregnant woman each had a urinary tract infection; a 78-year-old Vietnamese man had nosocomial aspiration pneumonia in which K. pneumoniae was considered a pathogen; and a 34-year-old African American woman who was receiving mechanical ventilation had sputum transiently colonized with K. pneumoniae.
This case series confirms that the clinical syndrome of K. pneumoniae bacteremia and primary liver abscess has emerged in Alameda County. Other sites of infection included kidney, brain, lung, pleural space, neck, and mediastinum, as reported in Asia (1–4). Although K. pneumoniae infections are predominantly a community-associated phenomenon, nosocomial infections as we observed have been reported (8). Death reflected underlying disease rather than K. pneumoniae infection in this study. We present evidence that hypermucoviscous K. pneumoniae also can behave as a colonizer or low-virulence pathogen, as manifested in our patients with urinary tract infection, sputum colonization, and aspiration pneumonia.
Our K. pneumoniae isolates appear similar to those from Asia (5) with respect to in vitro susceptibility, capsule serotypes, and magA and rmpA genes. Most of our patients were Asian but of widely dispersed origin and without recent travel to Asia. The number of thrombotic complications in this series is striking. Such complications appear not to have been noted in the literature, and this finding requires further investigation. Our data show the emergence of hypermucoviscous K. pneumoniae in northern California and suggest that it might be unrecognized elsewhere in North America.
Acknowledgment
We gratefully acknowledge the clinical microbiology technologists at Alameda County Medical Center and St. Rose Hospital who enthusiastically found and evaluated the isolates. Genotyping was kindly performed by the Microbial Diseases Laboratory, California Department of Public Health.
References
- Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscesses. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151:1557–9. DOIPubMedGoogle Scholar
- Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26:1434–8. DOIPubMedGoogle Scholar
- Lee SS, Chen YS, Tsai HC, Wann SR, Lin HH, Huang CK, Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscesses. Clin Infect Dis. 2008;47:642–50. DOIPubMedGoogle Scholar
- Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13:986–93.PubMedGoogle Scholar
- Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199:697–705. DOIPubMedGoogle Scholar
- McIver C, Janda J. Pathophysiology and laboratory identification of emerging hepatovirulent Klebsiella pneumoniae. Clin Microbiol Newsl. 2008;30:127–31. DOIGoogle Scholar
- Frazee BW, Hansen S, Lambert L. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med. 2009;53:639–42. DOIPubMedGoogle Scholar
- Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42:1351–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 16, Number 9—September 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Robert McCabe, 1531 Gilboa Dr, Walnut Creek, CA 94598, USA
Top