Volume 17, Number 10—October 2011
Dispatch
Yellow Fever Virus Vaccine–associated Deaths in Young Women1
Cite This Article
Citation for Media
Abstract
Yellow fever vaccine–associated viscerotropic disease is a rare sequela of live-attenuated virus vaccine. Elderly persons and persons who have had thymectomies have increased susceptibility. A review of published and other data suggested a higher than expected number of deaths from yellow fever vaccine–associated viscerotropic disease among women 19–34 years of age without known immunodeficiency.
Yellow fever virus (YFV) vaccine had been considered the safest of the live-virus vaccines. Rare neurologic adverse events, called yellow fever vaccine–associated neurotropic disease (YEL-AND), have long been recognized but are seldom fatal. However, in 2001, the vaccine was found to cause a serious, frequently fatal, multisystemic illness, called yellow fever vaccine–associated viscerotropic disease (YEL-AVD), which resembles the illness it was designed to prevent (1–3). According to reports from the Vaccine Adverse Event Reporting System (VAERS) (www.vaers.hhs.gov), the frequency of YEL-AVD in US vaccinees was 0.4 per 100,000 doses of vaccine administered (4).
Elderly persons (4) and patients who have undergone thymectomies secondary to thymoma (5) are recognized as groups at risk for YEL-AVD. However, several case reports of YEL-AVD in young women raise concern that women of childbearing age might also be at increased risk (6–10).
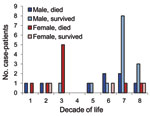
To investigate the possibility of age- and sex-specific risk groups, a comprehensive YEL-AVD dataset (Table 36-30 in 11), was analyzed (Figure). This dataset has the advantage of having been compiled with information that is not otherwise publicly available: data from the Centers for Disease Control and Prevention (Atlanta, GA, USA), patient charts, and vaccine manufacturers (T.P. Monath, pers. comm.).
Two concentrations of cases were evident: cases in men >60 years of age who survived and in women 19–34 years of age who died. Although selection bias may have influenced the cases reported, the sex-specific survival rates for these 2 age groups statistically differed: 21% (3/14) versus 0% (0/6) (p = 0.002 by Fisher exact test). In addition to the surprisingly low case-fatality rate for elderly men, only 2 of the 4 patients who had undergone thymectomy and had YEL-AVD died.
Searches for additional YEL-AVD cases among women of childbearing age (15–44 years) and of comparably aged men included review of published cases through PubMed (www.ncbi.nlm.nih.gov/sites/entrez) and reports from the ProMED Web site (http://apex.oracle.com/pls/otn/f?p=2400:1000:). In follow-up of a ProMED listing, 1 case was supplied by Bio-Manguinhos (Rio de Janeiro, Brazil), a producer of YFV vaccine. VAERS also was searched. Information was sought from authors of case reports. Cases listed in VAERS were excluded if another explanation for the adverse event was evident in the case description or if they contained insufficient information to classify the event as YEL-AVD.
A total of 9 fatal cases of YEL-AVD in young adults, all women, were found (Table). Six cases were included in the report by Monath et al. (11), and 3 cases were found through the author’s search. The eldest of the 9 case-patients was 34 years of age. One case listed by Monath et al. occurred in 1975 and was originally thought to be yellow fever but was documented as vaccine-related ≈2 decades later (13). This patient’s age is not known, but she was reported to be a young woman (P. Vasconcelos, pers. comm.).
Three fatal cases of possible YEL-AVD among young women reported in VAERS were excluded from the Table because information was insufficient to document the diagnosis. Two other cases of suspected YEL-AVD, 1 each in an 18-year-old man and a 24-year-old woman, occurred outside the United States. Hence, these patients could have come from regions where yellow fever was endemic and thus might have had wild-type yellow fever.
Also excluded from the Table are 2 cases reported in the published literature: 1 in a 23-year-old woman with a partial C4 deficiency and discoid lupus erythematosis hospitalized with severe YEL-AND and YEL-AVD who survived (14) and 1 in a 43-year-old woman with systemic lupus erythematosus who died (10). The first was excluded because she survived, had clinical features that included YEL-AND, and had known immunodeficiency. For the second patient, a history of disseminated lupus erythematosis, the relatively long interval (30 days) until death (in contrast to the 9–14 days in the other women), and her older age suggest that her susceptibility to the vaccine differed from those listed in the Table.
In several investigations of YEL-AVD cases, extensive sequence analyses did not indicate any substantial evidence of reversion of the vaccine to virulence (1,10). Two varieties of YFV vaccine are available: the 17DD vaccine produced in Brazil and used in South America and the 17D-204 vaccine (YF-Vax, Sanofi Pasteur, Swiftwater, PA, USA; and Stamaril, Sanofi Pasteur, Lyon, France) used elsewhere. Six cases listed in the Table occurred in 17DD vaccine recipients in South America, and 3 occurred in persons who received 17D-204 as prospective travelers.
The limited racial information available indicates that cases were not confined to persons of any particular racial group. Of 3 case-patients for whom racial information was available, 1 each was described as Caucasian (8), black (2), and of Pacific Islander ancestry (7).
Despite the known association of thymectomy with YEL-AVD, the only observation on possible thymic disease in the reports of the 9 cases is the statement that, at autopsy of a 22-year-old woman from the United States, the thymus was replaced by fat (Table 36-30 in 11). However, the accuracy of the finding should be considered in the context that, at surgery, experienced cardiothoracic surgeons may have difficulty in distinguishing thymus from adipose tissue (R.L. Berger, pers. comm.) and that the thymus was not examined histologically (R.V. Ridenour, III, pers. comm.). Because the thymus may be difficult to separate from surrounding adipose tissue and is infrequently a source of disease, pathologists, at least in the United States, do not routinely examine it histologically at autopsy (I. Argani, pers. comm.). Thymic deficiencies such as Sutton thymic dysplasia (fatal viral infection in young women with a dysplastic thymus) (15) have yet to be excluded.
Although accurate denominators are not available for calculating age- and sex-specific incidence of YEL-AVD, the number of fatal YEL-AVD cases among women of childbearing age appears to be higher than expected. Further investigation should include ascertainment of family history; exploration of contraceptive medications or occult pregnancy as possible predisposing factors; examination of the thymus at postmortem, including thymus weight and histology; further evaluation of possible complement defects; and evaluation of any associations with autoimmune disease.
Dr Seligman is a research professor in the Department of Microbiology and Immunology, New York Medical College. His current research interests include flaviviruses with emphasis on the safety of flavivirus vaccines.
Acknowledgment
The author thanks F.H. Moy for statistical assistance and J. Dinardi for computer aid with the figure. R. Menezes-Martins kindly provided data on a previously unreported case.
References
- Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J, Fever and multisystem organ failure associated with 17D–204 yellow fever vaccination: a report of four cases. Lancet. 2001;358:98–104. DOIPubMedGoogle Scholar
- Vasconcelos PF, Luna EJ, Galler R, Silva LJ, Coimbra TL, Barros VL, Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358:91–7. DOIPubMedGoogle Scholar
- Chan RC, Penney DJ, Little D, Carter IW, Roberts JA, Rawlinson WD. Hepatitis and death following vaccination with 17D–204 yellow fever vaccine. Lancet. 2001;358:121–2. DOIPubMedGoogle Scholar
- Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–82. DOIPubMedGoogle Scholar
- Barwick R. History of thymoma and yellow fever vaccination. Lancet. 2004;364:936. DOIPubMedGoogle Scholar
- Vasconcelos PF, Bryant JE, da Rosa TP, Tesh RB, Rodrigues SG, Barrett AD. Genetic divergence and dispersal of yellow fever virus, Brazil. Emerg Infect Dis. 2004;10:1578–84.PubMedGoogle Scholar
- Gerasimon G, Lowry K. Rare case of fatal yellow fever vaccine–associated viscerotropic disease. South Med J. 2005;98:653–6. DOIPubMedGoogle Scholar
- Doblas A, Domingo C, Bae HG, Bohorquez CL, de Ory F, Niedrig M, Yellow fever vaccine–associated viscerotropic disease and death in Spain. J Clin Virol. 2006;36:156–8. DOIPubMedGoogle Scholar
- Belsher JL, Gay P, Brinton M, DellaValla J, Ridenour R, Lanciotti R, Fatal multiorgan failure due to yellow fever vaccine–associated viscerotropic disease. Vaccine. 2007;25:8480–5. DOIPubMedGoogle Scholar
- Whittembury A, Ramirez G, Hernandez H, Ropero AM, Waterman S, Ticona M, Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. 2009;27:5974–81. DOIPubMedGoogle Scholar
- Monath TP, Cetron MS, Teuwen DE. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, 5th ed. Philadelphia: Saunders; 2008. p. 959–1055.
- Struchiner CJ, Luz PM, Dourado I, Sato HK, Aguiar SG, Ribeiro JG, Risk of fatal adverse events associated with 17DD yellow fever vaccine. Epidemiol Infect. 2004;132:939–46. DOIPubMedGoogle Scholar
- Engel AR, Vasconcelos PF, McArthur MA, Barrett AD. Characterization of a viscerotropic yellow fever vaccine variant from a patient in Brazil. Vaccine. 2006;24:2803–9. DOIPubMedGoogle Scholar
- Silva ML, Espirito-Santo LR, Martins MA, Silveira-Lemos D, Peruhype-Magalhaes V, Caminha RC, Clinical and immunological insights on severe, adverse neurotropic and viscerotropic disease following 17D yellow fever vaccination. Clin Vaccine Immunol. 2010;17:118–26. DOIPubMedGoogle Scholar
- Sutton AL, Smithwick EM, Seligman SJ, Kim DS. Fatal disseminated herpesvirus hominis type 2 infection in an adult with associated thymic dysplasia. Am J Med. 1974;56:545–53. DOIPubMedGoogle Scholar
Figure
Table
Cite This Article1Data previously presented at a National Institutes of Health–supported Northeast Biodefense Center conference, New Paltz, New York, USA, November 2, 2010.
Table of Contents – Volume 17, Number 10—October 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Stephen J. Seligman, Department of Microbiology and Immunology, New York Medical College, Valhalla, NY 10595, USA
Top