Volume 17, Number 5—May 2011
Letter
Avian Malaria Deaths in Parrots, Europe
Figure
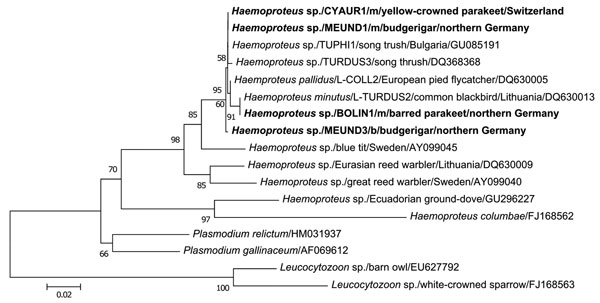
Figure. Phylogenetic relationships based on alignment of 479 bp of the cytochrome b gene of Haemoproteus spp. isolated from megalomeronts (m) of infected muscles and blood (b) of parrots with related hematozoan parasites in GenBank and the database MalAvi (http://mbio-serv4.mbioekol.lu.se/avianmalaria; 10). Nucleotide distance values of the maximum likelihood phylogenetic tree were calculated under the HKY substitution model. New sequences of Haemoproteus spp. from parrots of this study are shown in boldface. Two distinct species of the genus Leucocytozoon served as outgroup of the phylogenetic tree. The branch lengths are proportional to the degree of inferred evolutionary change as shown by the scale bar, and the numbers indicate bootstrap values (1,000 replicates). While the cytochrome b sequences CYAUR1, MEUND1, and BOLIN1, respectively, found matching sequences, MEUND3 showed closest sequence similarities with Haemoproteus spp. of the lineage COLL2, which depict a wider host breadth among songbirds (http://mbio-serv4.mbioekol.lu.se/avianmalaria). The isolates of Haemoproteus spp. from psittacine birds were deposited into GenBank under accession nos. HQ398207–HQ398212.
References
- Atkinson CT, van Riper C III. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In Loye JE, Zuk M, editors. Bird–parasite interactions. Ecology, evolution, and behavior. New York: Oxford University Press; 1991. p. 19–48.
- Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008;47:261–73. DOIPubMedGoogle Scholar
- van Riper C III, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327–44. DOIGoogle Scholar
- Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proc Biol Sci. 2004;271:1363–70. DOIPubMedGoogle Scholar
- Bennett GF, Peirce MA, Ashford RW. Avian haematozoa: mortality and pathogenicity. J Nat Hist. 1993;27:993–1001. DOIGoogle Scholar
- Stidworthy MF, Greenwood AG. Deaths in aviary birds associated with protozoal megaloschizonts. Vet Rec. 2006;159:606. DOIPubMedGoogle Scholar
- Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. DOIPubMedGoogle Scholar
- Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G. Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol. 2006;92:1319–24. DOIPubMedGoogle Scholar
- Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proc Biol Sci. 2002;269:885–92. DOIPubMedGoogle Scholar
- Bensch S, Hellgren O, Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resources. 2009;9:1353–8. DOIPubMedGoogle Scholar