Volume 17, Number 6—June 2011
Letter
Saffold Cardioviruses in Children with Diarrhea, Thailand
Cite This Article
Citation for Media
To the Editor: Cardioviruses currently consist of at least 3 viruses: Theiler murine encephalomyocarditis virus, encephalomyocarditis virus, and Saffold virus (SAFV) (1–4). Saffold cardiovirus in the family Picornaviridae was isolated and identified from fecal specimens of a child with fever of unknown origin in the United States (3).
Several reports have documented the presence of SAFV in fecal samples and respiratory secretions (5–10). However, it is not clear whether SAFV is associated with any disease, including gastroenteritis in humans, and epidemiologic data for SAFV are limited. We report an epidemiologic survey of SAFV in children hospitalized with diarrhea in Chiang Mai, Thailand.
A total of 150 fecal specimens were obtained from children hospitalized with acute gastroenteritis in Chiang Mai during January–December 2007. Patient ages ranged from >1 to 5 years. SAFV in fecal specimens was detected by using a nested PCR and primers specific for the virus 5′ untranslated region (7). A negative control was also included to monitor any contamination that might have occurred during the PCR.
SAFVs detected were further analyzed by amplification of the viral protein (VP) 1 gene (6,9,10) and direct sequencing of the VP1 PCR amplicon by using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). VP1 sequence was compared with VP1 sequences of reference strains available in the National Center for Biotechnology Information (Bethesda, MD, USA). Phylogenetic and molecular evolutionary analyses were conducted by using MEGA4 (www.megasoftware.net). Nucleotide sequences of SAFV strains described were deposited in GenBank under accession nos. HQ668170–HQ668173.
Four (2.7%) of 150 specimens were positive for SAFV (CMH023/2007, CMH038/2007, CMH045/2007, and CMH143/2007). Two of these specimens (CMH023/2007 and CMH038/2007) were obtained in February 2007, one (CMH045/2007) in March 2007, and 1 (CMH143/2007) in November 2007. Co-infections with other viruses were detected in all 4 samples. Two specimens (CMH023/2007and CMH045/2007), were co-infected with noroviruses GII/16 and GII/4 genotypes, respectively. One SAFV-positive sample (CMH038/2007) was co-infected with a group A rotavirus G1P[8] genotype, and another (CMH143/2007) was co-infected with human parechovirus.
All SAFV-positive specimens were further amplified for the VP1 gene to determine their phylogenetic lineages and genetic relationships with other SAFV reference strains. When we used 3 sets of primers used in other studies (6,9,10) for amplification of the VP1 gene, this gene was amplified only by the primer set reported by Itagaki et al. (10).
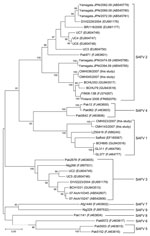
Analysis of partial VP1 sequences (369 nt) of 4 SAFV strains showed that strains CMH023/2007 and CMH143/2007 were highly conserved (nt sequence identities >97%). These 2 SAFV strains were most closely related to the prototype strain of SAFV1 (EF165067) isolated in the United States (nt sequence identity range 87.6%–88.9%) and SAFV strains from China (LZ50419, BCH895, GL311, and GL377) (Figure). In addition, the other 2 SAFVs identified in the present study (CMH038/2007 and CMH045/2007) were identical to each other and closely related to SAFV2 strains from China (BCHU79, BCHU353) and Finland (Finland 2008, FIN08–13B) (nt sequence identity range 94.8%–95.6%). Phylogenetic analysis showed that CMH038/2007 and CMH045/2007 were clustered within the SAFV2 lineage (Figure).
The 4 strains of SAFV were isolated from children with acute gastroenteritis who were co-infected with other viral pathogens (norovirus, group A rotavirus, and human parechovirus). Therefore, we could not determine whether SAFVs identified in this study were associated with acute gastroenteritis. The detection rate for SAFV in children with acute gastroenteritis (2.7%) in our study was consistent with that in a study in Beijing, People’s Republic of China (3.2%) (9).
Phylogenetic analysis of the VP1 region demonstrated that 2 SAFV lineages (SAFV1 and SAFV2) were circulating in Chiang Mai, Thailand. Further extensive epidemiologic surveillance of SAFV in other areas may provide a better understanding of the distribution, heterogeneity, and association of SAFV with enteric diseases in humans.
Acknowledgment
This study was supported by the Endowment Fund for Medical Research, Faculty of Medicine, Chiang Mai University, Thailand, and in part by Grants-in-Aid from the Ministry of Education and Sciences and the Ministry of Health, Labor and Welfare, Japan.
References
- LaRue R, Myers S, Brewer L, Shaw DP, Brown C, Seal BS, A wild-type porcine encephalomyocarditis virus containing a short poly(C) tract is pathogenic to mice, pigs, and cynomolgus macaques. J Virol. 2003;77:9136–46. DOIPubMedGoogle Scholar
- Liang Z, Kumar AS, Jones MS, Knowles NJ, Lipton HL. Phylogenetic analysis of the species Theilovirus: emerging murine and human pathogens. J Virol. 2008;82:11545–54. DOIPubMedGoogle Scholar
- Jones MS, Lukashov VV, Ganac RD, Schnurr DP. Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol. 2007;45:2144–50. DOIPubMedGoogle Scholar
- Drexler JF, Baumgarte S, Luna LK, Stöcker A, Almeida PS, Ribeiro TC, Genomic features and evolutionary constraints in Saffold-like cardioviruses. J Gen Virol. 2010;91:1418–27. DOIPubMedGoogle Scholar
- Abed Y, Boivin G. New Saffold cardioviruses in 3 children, Canada. Emerg Infect Dis. 2008;14:834–6.PubMedGoogle Scholar
- Chiu CY, Greninger AL, Kanada K, Kwok T, Fischer KF, Runckel C, Identification of cardioviruses related to Theiler’s murine encephalomyelitis virus in human infections. Proc Natl Acad Sci U S A. 2008;105:14124–9. DOIPubMedGoogle Scholar
- Drexler JF, Luna LK, Stöcker A, Almeida PS, Ribeiro TC, Petersen N, Circulation of 3 lineages of a novel Saffold cardiovirus in humans. Emerg Infect Dis. 2008;14:1398–405. DOIPubMedGoogle Scholar
- Blinkova O, Kapoor A, Victoria J, Jones M, Wolfe N, Naeem A, Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J Virol. 2009;83:4631–41. DOIPubMedGoogle Scholar
- Ren L, Gonzalez R, Xiao Y, Xu X, Chen L, Vernet G, Saffold cardiovirus in children with acute gastroenteritis, Beijing, China. Emerg Infect Dis. 2009;15:1509–11. DOIPubMedGoogle Scholar
- Itagaki T, Abiko C, Ikeda T, Aoki Y, Seto J, Mizuta K, Sequence and phylogenetic analyses of Saffold cardiovirus from children with exudative tonsillitis in Yamagata, Japan. Scand J Infect Dis. 2010;42:950–2. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 17, Number 6—June 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Niwat Maneekarn, Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
Top