Volume 17, Number 7—July 2011
Dispatch
Natural Burkholderia mallei Infection in Dromedary, Bahrain
Cite This Article
Citation for Media
Abstract
We confirm a natural infection of dromedaries with glanders. Multilocus variable number tandem repeat analysis of a Burkholderia mallei strain isolated from a diseased dromedary in Bahrain revealed close genetic proximity to strain Dubai 7, which caused an outbreak of glanders in horses in the United Arab Emirates in 2004.
Glanders, a World Organisation for Animal Health (OIE)–listed disease, is a contagious, life-threatening disease of equids caused by the gram-negative bacterium Burkholderia mallei (1). Although eliminated in western Europe, glanders remains endemic to several Asian, African, and South American countries. It recently reappeared in Pakistan and Brazil in 2008 and 2009, respectively, and appeared for the first time in Kuwait and Bahrain in 2010 (2,3).
Natural B. mallei infections are known to occur in various mammals (e.g., cats, bears, wolves, and dogs). Camels are also susceptible to B. mallei, as experimental infection has demonstrated (4,5). We report a natural infection of dromedaries (Camelus dromedarius).
An outbreak of glanders is ongoing in equids in Bahrain (6). Most of the reported cases were found in Saar and Shakhoura in the Northern governorate. Samples from 4,843 horses and 120 donkeys were sent to the OIE Reference Laboratory at the Central Veterinary Research Laboratory in Dubai, United Arab Emirates. Of these samples, 45 horses with clinical signs consistent with glanders were positive by complement fixation test and were euthanized along with 4 donkeys that also had positive test results. In addition to horses and donkeys, dromedaries showed clinical signs of glanders, but B. mallei infection has not yet been confirmed. Here we provide evidence for a B. mallei infection in 1 of the diseased dromedaries.
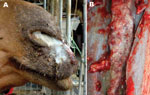
On a small private farm, 2 of 7 horses had positive serologic reactions and showed typical clinical signs of glanders. On the same premises, 6 dromedaries were kept several meters away from the sick horses in a separate enclosure. Three dromedaries that showed clinical signs of glanders, including severe mucopurulent discharge from both nostrils (Figure 1, panel A), fever, emaciation, and fatigue, died. One of these dromedaries underwent necropsy. Serum samples from this dromedary tested positive for glanders with both the OIE-acknowledged complement fixation test (titer 10+++) and with the Central Veterinary Research Laboratory–developed in-house competitive ELISA (7) with an inhibition of 57%.
An EDTA blood sample was incubated for 11 days in a blood culture system (Oxoid, Cambridge, UK) until it became positive. This fluid was then cultured on sheep blood agar at 37°C for 72 h. The isolate stained poorly gram-negative, was rod shaped, and tested oxidase positive. Suspected B. mallei colonies were analyzed with the API 20 NE-test (bioMérieux, Marcy l’Etoile, France) and were positive for nitrate, glucose assimilation, arginine dehydrolase (after 4 days of incubation), N–acetyl glucosamine, and potassium gluconate. The API 20 NE-test identified the colonies as B. mallei because the same API ID number (1140504) occurred as in the previously isolated Dubai 7 strain (1).
During necropsy, typical glanderous lesions in the lung, choanae, and nasal septae were observed. Golf ball–sized reddish-gray nodules resembling tubercles with a central gray necrotic zone were detected in the lungs. In the choanae and nasal septae, stellate scars, ulcers, and honeycomb necrotic patches covered with yellow pus (Figure 1, panel B) were seen. Glanderous lesions were absent from other organs.
The presence of B. mallei in lung and choanae specimens was examined by using standard culturing techniques as described by Wittig et al. (1). For bacterial growth, sheep blood agar plates were incubated at 37°C for 72 h. B. mallei was directly isolated from the pus, which had accumulated in the choanae, but not from nasal and eye swabs and not from the lung lesions. However, the tissue samples were stored at –20°C for >20 days before incubation.
For molecular analysis, cultivated bacteria were resuspended in saline and inactivated at 98°C for 20 min. Total DNA was extracted by using the DNA-purification Kit (QIAGEN, Hilden, Germany). Sequence analysis of the 16S rRNA (1,400 pb) gene displayed the B. mallei–specific single nucleotide polymorphism that differentiates B. mallei from B. pseudomallei (8) (not shown). B. mallei was further confirmed by multilocus sequence typing displaying the B. mallei–specific sequence type 40 (alleles 1, 12, 3, 4, 1, 18, 1), as previously described by Godoy et al. (9).
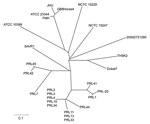
Multilocus variable number tandem repeat analysis based on 23 different loci (10) was used for further subtyping through sequencing of the variable number tandem repeat regions (Table A1). Phylogenetic analysis of these data was performed as described by Hornstra et al. (10) and compared with existing B. mallei strains (Figure 2). In this analysis, the strain (THSK2) isolated from the dromedary clustered with B. mallei strain Dubai 7 (Figure 2) that had been isolated from a horse in the United Arab Emirates (11).
Old World camels, the dromedary (C. dromedarius), and the Bactrian camel (C. bactrianus) are susceptible to B. mallei (glanders) and B. pseudomallei (melioidosis) infection (12,13). However, reports of B. mallei infection in dromedaries have described artificial infections (4,5). We report natural B. mallei infection in a dromedary that occurred during a glanders outbreak in horses.
Clinical signs as well as gross pathologic and microscopic lesions of the diseased dromedary were similar to changes seen in equids. These changes were dominated by severe mucopurulent nasal discharge, nodules and ulcers with pus in the choanae and nasal septae, and granulomas in the lungs that resembled tubercle lesions (pseudo tubercles).
B. mallei was isolated from venous blood, indicating septicemia. The pathogen was also directly isolated from the pus, which had accumulated in the choanae, but not from nasal and eye swabs and, unexpectedly not from the lung lesions. A possible explanation for the failure to isolate B. mallei from the nasal swabs was the heavy growth of various other contaminating bacteria because no selective culture medium exists for B. mallei. It could also be explained by storage of the samples at –20°C for >20 days, which probably destroyed the bacteria.
The genetic relatedness of the strain isolated from the dromedary to the strain isolated in 2004 from horses in the United Arab Emirates suggests that this strain might be endemic to this region. It also appears to be genetically distinct from a recent outbreak in Pakistan, demonstrating the persistence of multiple strains on a larger geographic scale. Isolation of this pathogen from both camels and horses poses new challenges to the international trade of equids from and to countries where camels are raised.
Dr Wernery is scientific director of the Central Veterinary Research Laboratory, Dubai, United Arab Emirates. His research interests include infectious diseases, such as glanders, meliodosis, blue tongue, West Nile, and others, in camelids. He is also interested in the medicinal properties of camel milk.
Acknowledgments
We thank Rahime Terzioglou and Astrid Thomas for excellent technical assistance.
H.H. was supported by the Science & Technology Directorate, US Department of Homeland Security, under award 2010-ST-1080-000015.
References
- Wittig MB, Wohlsein P, Hagen RM, Al Dahouk S, Tomaso H, Scholz HC, Glanders—a comprehensive review [in German]. Dtsch Tierarztl Wochenschr. 2006;113:323–30.PubMedGoogle Scholar
- Roberts H, Lopez M, Hancock R. International disease monitoring, April to June. Vet Rec. 2010;167:192–5. DOIPubMedGoogle Scholar
- Wernery U. Glanders. In Mair TS, Hutchinson RE, editors. Infectious diseases of the horse. Fordham (UK): Equine Veterinary Journal, Ltd. 2009. p. 253–260.
- Samartsev AA, Arbuzov PN. The susceptibility of camels to glanders, rinderpest and bovine pleuropneumonia. Veterinaria (Mosk). 1940;4:59–63.
- Curasson G. Le chameau et ses maladies. Paris: Vigot Frères; 1947. p. 86–8.
- Equine disease surveillance, April to June 2010. Vet Rec. 2010;167:598–601.PubMed DOIPubMedGoogle Scholar
- Sprague LD, Zachariah R, Neubauer H, Wernery R, Joseph M, Scholz HC, Prevalence dependent use of serological tests for diagnosing glanders in horses. BMC Vet Res. 2009;5:1–6.PubMed DOIPubMedGoogle Scholar
- Gee JE, Sacchi CT, Glass MB, De BK, Weyant RS, Levett PN, Use of 16S rRNA gene sequencing for rapid identification and differentiation of Burkholderia pseudomallei and B. mallei. J Clin Microbiol. 2003;41:4647–54. DOIPubMedGoogle Scholar
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–79. DOIPubMedGoogle Scholar
- Hornstra H, Pearson T, Georgia S, Liguori A, Dale J, Price E, Molecular epidemiology of glanders, Pakistan. Emerg Infect Dis. 2009;15:2036–9. DOIPubMedGoogle Scholar
- Scholz HC, Joseph M, Tomaso H, Al Dahouk S, Witte A, Kinne J, Detection of the reemerging agent Burkholderia mallei in a recent outbreak of glanders in the United Arab Emirates by a newly developed fliP-based polymerase chain reaction assay. Diagn Microbiol Infect Dis. 2006;54:241–7. DOIPubMedGoogle Scholar
- Wernery R, Kinne J, Hayden-Evans J. Ul haq A. Melioidosis in a seven year old camel. A new disease in the United Arab Emirates (UAE). Journal of Camel Practice and Research. 1997;4:141–3.
- Wernery U, Kaaden O-R. Infectious diseases in camelids, 2nd ed. Berlin: Blackwell Science; 2002. p. 91–7.
Figures
Table
Cite This ArticleTable of Contents – Volume 17, Number 7—July 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Holger C. Scholz, Bundeswehr Institute of Microbiology, Neuherbergstr 11, 80937 Munich, Germany
Top