Volume 17, Number 9—September 2011
Research
Central Venous Catheter–associated Nocardia Bacteremia in Cancer Patients
Cite This Article
Citation for Media
Abstract
Central venous catheters, often needed by cancer patients, can be the source of Nocardia bacteremia. We evaluated the clinical characteristics and outcomes of 17 cancer patients with Nocardia bacteremia. For 10 patients, the bacteremia was associated with the catheter; for the other 7, it was a disseminated infection. N. nova complex was the leading cause of bacteremia. Nocardia promoted heavy biofilm formation on the surface of central venous catheter segments tested in an in vitro biofilm model. Trimethoprim- and minocycline-based lock solutions had potent in vitro activity against biofilm growth. Patients with Nocardia central venous catheter–associated bloodstream infections responded well to catheter removal and antimicrobial drug therapy, whereas those with disseminated bacteremia had poor prognoses.
Nocardiae are partially acid-fast, aerobic, gram-positive, branching filamentous bacteria that are found ubiquitously in soil, fresh water, and marine water (1,2). Nocardia spp. cause serious pulmonary infections (with occasional brain abscesses) in immunocompromised patients, primarily those with cell-mediated immunity abnormalities (2,3). Nocardiosis most commonly occurs after the organism has been introduced into the respiratory tract, but it may be acquired through direct inoculation into the skin (4). However, Nocardia bacteremia is rarely reported, even for severely immunocompromised patients with underlying malignancies (5,6).
Our first objective was to identify the clinical characteristics of Nocardia bacteremia and compare the clinical profiles and outcomes for patients with Nocardia bacteremia associated with central venous catheters (CVCs), also called central line–associated bloodstream infections (CLABSIs), with those of patients with disseminated Nocardia bacteremia. Our second objective was to determine whether Nocardia bacteria could form a biofilm on CVCs in a laboratory model and whether biofilm growth could be prevented with the use of antimicrobial lock solutions.
Clinical Characteristics
By searching the microbiology laboratory database at The University of Texas MD Anderson Cancer Center (Houston, TX, USA) from January 1998 through March 2010, we retrospectively identified 134 episodes of nocardiosis of any sources. Nocardia bacteremia was reported for 17 patients; 5 of these cases have been reported by Torres et al. (7). In addition, 2 of these 5 patients had catheter-related bloodstream infections (CRBSIs), reported by Kontoyiannis et al. (8). Pertinent data from patients’ medical records were abstracted, including demographic characteristics, underlying malignancies, hematopoietic stem cell transplantation, graft-versus-host disease, clinical presentation, laboratory test and imaging study results, concomitant infections, antimicrobial therapy types and durations, hospital and intensive care unit stays, 72-hour and 7-day responses, and patient outcomes at 3-month follow-up.
Definitions
Neutropenia was defined as an absolute neutrophil count <500 cells/mm3 and lymphopenia as an absolute lymphocyte count <1,000 cells/mm3. Patients with at least 1 set of positive blood cultures for Nocardia bacteria were considered to have Nocardia bacteremia. CLABSIs were diagnosed according to Centers for Disease Control and Prevention (Atlanta, GA, USA) guidelines: a recognized pathogen cultured from >1 blood cultures (not including organisms considered common skin contaminants) and no apparent source of the bloodstream infection except the CVC whereby the CVC has been indwelling for >48 hours (9). Furthermore, CLABSIs were considered definite CRBSIs if at least 1 of the Infectious Disease Society of America definition criteria were also fulfilled: semiquantitative (>15 CFUs/catheter segment) or quantitative (>103 CFUs/catheter segment) catheter culture in which the same organism is isolated from the catheter segment and peripheral blood, or differential quantitative blood culture with simultaneous quantitative blood cultures from the CVC and peripheral blood with a ratio >3:1 (10). Disseminated (or secondary) Nocardia bacteremia was defined as the recovery of Nocardia bacteria from blood cultures and a non–catheter-related site (e.g., expectorated sputum, endotracheal aspiration, bronchoalveolar lavage, pleural effusion, lung tissue, or skin or brain biopsy samples) in the setting of clinical and radiographic evidence of organ involvement (pneumonia and skin lesions).
Nocardia spp. were identified on the basis of the appearance of colonies on routine media; species were identified by using a battery of biochemical tests and, after 2001, by 16S rDNA sequencing (11,12). Blood cultures and catheter tip samples were held for 7 days to ensure that no cases of Nocardia bacteremia were missed.
A broth microdilution MIC method had been used to perform susceptibility testing of Nocardia spp. according Clinical and Laboratory Standards Institute guidelines (13). Antimicrobial drug response had been defined as resolution or improvement of clinical manifestations and radiographic changes and negative microbiological findings.
Biofilm Formation
We used a modified Kuhn model of biofilm catheter colonization (14) to test N. nova complex and N. puris strains (that caused CLABSI in this study) for biofilm formation. Sterile polyurethane and silicone CVC segments were placed in 24-well tissue culture plates containing human donor plasma and incubated with shaking for 24 h at 37°C. The plasma was then replaced with 1 mL of 5.5 × 105 cells/mL inoculum of Nocardia strains. The Nocardia inoculum was grown in tryptic soy broth containing 10% fetal bovine serum and incubated with shaking for 24 h at 37°C. Organisms were tested in triplicate. The inoculated broth was removed, and CVC segments were washed with 1 mL of 0.9% sterile saline by shaking at 100 rpm for 30 min at 37°C. The CVC segments were transferred into a tube containing 5 mL of sterile 0.9% saline and sonicated for 15 min to disrupt any biofilm. The resulting solution was then cultured and quantified by making serial dilutions in 0.9% sterile saline and spreading them on trypticase soy agar plates with 5% sheep blood. All plates were inverted and incubated for 48 h at 37°C. The experiment was repeated 2 times.
Antimicrobial Lock Solutions
To determine whether antimicrobial lock solutions prevented the biofilm growth of Nocardia organisms, we used the silicone disk biofilm colonization model, as described (14). After washing the silicone disks with 0.9% sterile saline by shaking them for 30 min at 37°C, we transferred the disks into new 24-well tissue culture plates containing Mueller-Hinton broth (control) or the drug solution to be tested. Drug solutions included 10 mg/mL trimethoprim/sulfamethoxazole and 100 U heparin; a triple combination of 10 mg/mL trimethoprim, 30 mg/mL EDTA, and 25% ethanol; and 3 mg/mL minocycline, 30 mg EDTA, and 25% ethanol. Triple combinations of minocycline, EDTA, and 25% ethanol lock solutions were used because previous data showed that such combinations effectively eradicate bacterial organisms embedded in biofilm (15). After 2 h of incubation at 37°C, the disks were placed in 5 mL of 0.9% saline and sonicated for 15 min. Finally, they were vortexed for 5 seconds, and 100 µL of liquid from each disk was serially diluted and spread on trypticase soy agar plates with 5% sheep blood for quantitative culture. Plates were then inverted and incubated for 48–72 h at 37°C, and colony growth was quantified. The experiment was repeated 2 times.
Electronic Microscopy and Confocal Scanning
To verify the quantitative results, we used scanning electron microscopy to examine biofilm formation of N. nova complex on silicone CVC surfaces. We used silicone CVCs tested in the in vitro colonization model outlined above. Catheters were fixed with 2% glutaraldehyde, followed by osmium tetraoxide, tannic acid, and uranyl acetate as described (16). A series of ethanol dehydration steps followed, and the prepared samples were sputter coated with Au-Pd (60:40) and viewed with a Philips model XL3C scanning electron microscope (Philips Research, Eindhoven, the Netherlands). Confocal scanning laser microscopy was performed with a Leica TCSNT confocal microscope (Leica, Heidelberg, Germany). Objectives used for confocal laser microscope imaging were 100×1.4 N.A. Oil Plan Apo and 63×0.7 N.A. Plan Fluotar.
Statistical Analyses
To compare the characteristics of Nocardia CLABSI and disseminated Nocardia bacteremia patients, we used SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA). We determined differences between categorical variable frequencies by using the χ2 or Fisher exact tests and compared continuous variables by using the Wilcoxon rank-sum test. All tests were 2-sided and had a maximum significance level of 0.05.
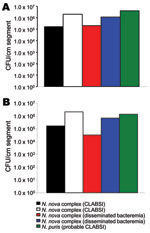
The demographic characteristics and outcomes of the 17 patients in the study are shown in Table 1. All patients had had CVCs inserted before examination and collection of blood for culture. Ten (59%) patients had CLABSIs (Table 2); the remaining 7 (41%) had disseminated (secondary) Nocardia bacteremia for which the respiratory tract (pneumonia) was the primary source. Concomitant infections, most commonly with cytomegalovirus, were noted for 10 (59%) patients. Hematologic malignancies were more common in patients with disseminated Nocardia bacteremia than with Nocardia CLABSIs (100% vs. 50%; p = 0.044). N. nova complex was the most commonly identified causative species for nocardemia (6 patients). Other causative species were N. asteroides complex (5 patients), N. veterana (2), N. brasiliensis (1), and N. puris (1). We found no significant differences in species distribution among patients with CLABSIs and disseminated bacteremia. Furthermore, by looking at the temporal distribution of cases of Nocardia bacteremia and CLABSI nocardemia, we noticed no cluster that would suggest we were dealing with an outbreak.
Patients with CLABSIs had shorter hospital stays than did those with disseminated bacteremia (median 5 days vs. 24 days, respectively; p = 0.01) and were more likely to experience a response to therapy within 72 hours (90% vs. 0%; p<0.001) or 7 days (90% vs. 29%; p = 0.035). Mortality rate at 3-month follow-up was also lower for patients with CLABSI (10% vs. 43%; p = 0.25).
All Nocardia isolates were susceptible to trimethoprim/sulfamethoxazole, amikacin, and linezolid. Susceptibility rates to ceftriaxone, clarithromycin, imipenem, minocycline, tobramycin, and amoxicillin and clavulanate were 91%, 87%, 85%, 55%, 42%, and 22%, respectively. All N. nova isolates were susceptible to clarithromycin. No resistance to minocycline was found, but 45% of isolates had intermediate susceptibility MICs. N. nova complex isolates showed resistance to amoxicillin and clavulanate and to gentamicin in 80% and 60% of cases, respectively, whereas 91% of all Nocardia species were resistant to ciprofloxacin.
All patients underwent CVC removal. The most commonly used drug was trimethoprim/sulfamethoxazole (14 patients), used alone or in combination with beta-lactams (8 patients with carbapenems and 2 with ceftriaxone), minocycline (5 patients), or amikacin (4 patients). A positive response occurred in 14 patients, and breakthrough Nocardia bacteremia occurred in 3 patients receiving trimethoprim/sulfamethoxazole prophylaxis (2 with CLABSIs and 1 with disseminated bacteremia).
Nocardia isolates adhered extensively to polyurethane and silicone CVCs and formed extensive biofilms (Figure 1). Quantitative biofilm cultures isolated ranged from 4.0 × 105 to 1.1 × 107 CFUs of tested Nocardia cells from biofilm matrices that adhered to polyurethane and silicone CVC surfaces. Furthermore, trimethoprim/sulfamethoxazole with heparin, minocycline, and EDTA in 25% ethanol, and trimethoprim and EDTA in 25% ethanol significantly decreased the biofilm biomasses on treated vs. control plates after a 2-hour exposure (p = 0.003), resulting in complete eradication of Nocardia spp. in biofilm on silicone disks (Figure 2).
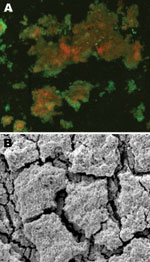
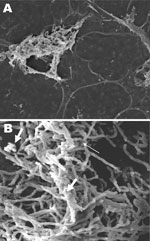
Electron and confocal scanning laser microscopic studies of the CVC tip in a patient with an N. nova CLABSI showed Nocardia spp. adhering to the surface and colonies of multilayered clusters embedded in biofilm matrix (Figure 3). Scanning electron microscopy studies of N. nova complex (disseminated Nocardia bacteremia) showed a heavy biofilm matrix covering filamentous clusters (Figure 4, panel A). N. nova complex (definite CLABSI) formed branching networks of filamentous bacteria encompassed in an intense heavy biofilm matrix (Figure 4, panel B).
We have documented that Nocardia spp. that cause clinical CLABSI also form heavy biofilm on the surfaces of polyurethane and silicone CVCs; that trimethoprim- and minocycline-based lock solutions eradicate adherent catheter-related Nocardia spp. in the biofilm matrix (thereby demonstrating Koch’s postulate as it relates to Nocardia CLABSI); and that Nocardia bacteremia in cancer patients can occur as CLABSI in 59% of cases. Nocardia CLABSI responds well to antimicrobial agents and CVC removal and is associated with a short hospital stay, whereas disseminated Nocardia bacteremia is associated with a poor prognosis.
Identification of Nocardia is more rapid, precise, and accurate with PCR and 16S rDNA sequencing than with standard phenotypic techniques (17–19). N. asteroides complex, which includes N. farcinica, N. nova, and N. asteroides sensu stricto, is the most commonly identified causative species in cancer patients (7). In our study, N. nova complex was responsible for 35% of Nocardia bacteremia episodes. N. nova is differentiated from other members of N. asteroides complex by DNA homologic characteristics; it is more appropriate to refer to N. nova as N. nova complex because it comprises 4 distinct species (12,17,20). Furthermore, the incidence of N. nova complex bacteremia may have been underestimated in our study because the species was not identified for 5 cases of infection with N. asteroides complex. N. nova complex strains may have higher tendencies toward CVC adherence, biofilm formation, and hematogenous spread.
Nocardia bacteremia is rare, even in severely immunocompromised patients with underlying malignancies (11). A 1998 review of the medical literature found only 36 cases of Nocardia bacteremia worldwide over 52 years (5). All Nocardia bacteremia patients in our study had indwelling CVCs before diagnosis and subsequently had catheters removed. Cancer patients depend immensely on vascular access devices, and CVCs may be the source and focus of Nocardia bacteremia, given the ubiquitous presence of these organisms in the environment, their possible acquisition through the skin, and their ability to adhere to CVCs through biofilm formation on the surface of catheters as shown in our study (Figure 1) (7,21,22).
Nocardia CLABSI cases, in contrast to disseminated Nocardia bacteremia cases, were associated with a favorable outcome. Catheter-related Nocardia bacteremia might be less likely to invade remote anatomical structures and form deep-seated infections. Disseminated Nocardia bacteremia, on the other hand, was associated with higher rates of admission to intensive care units, more frequent occurrence with hematologic malignancy, longer hospital stays, and lower antimicrobial therapy response rates. Factors that could have contributed to the poor outcome include involvement of the lungs and other organs; severity of the underlying disease (hematologic malignancy); and a higher rate of concomitant infections, most commonly with cytomegalovirus and invasive fungi (Table 1).
Although trimethoprim/sulfamethoxazole was uniformly active against Nocardia spp. and the most commonly used drug for Nocardia bacteremia, breakthrough Nocardia bacteremia occurred in 3 patients despite receipt of trimethoprim/sulfamethoxazole prophylaxis. Given that Nocardia spp. were shown in our model to form an antimicrobial drug–resistant multilayered biofilm matrix, in which they embed themselves, it is not surprising that 2 of the 3 cases of breakthrough Nocardia bacteremia that occurred during trimethoprim/sulfamethoxazole prophylaxis were CLABSIs. CVC biofilm colonization through formation of an antimicrobial drug–resistant matrix is the main factor in the pathogenesis of CRBSIs. The biofilm enables Nocardia spp. to protect themselves from the relatively low serum concentrations of antimicrobial drugs given orally and, hence, create a foothold from which they can invade the bloodstream through the surface of the CVC intravascular segment. Prophylactic antimicrobial drugs given orally are ineffective at breaking down the matrix and eradicating the bacteria, which poses a therapeutic and prophylactic challenge for cancer patients with a CVC. Furthermore, trimethoprim/sulfamethoxazole has been ineffective when used alone, especially against disseminated forms of nocardiosis (23).
Intraluminal antimicrobial lock therapy has been proposed for the prevention and treatment of CLABSIs (24–27). For antimicrobial lock therapy, the catheter lumen is filled with 2–4 mL of antimicrobial solution at a concentration 100- to 1,000-fold higher than the MIC of the drug or its usual target systemic concentration; to eradicate the organisms embedded in the intraluminal biofilm, the solution is then allowed to dwell (lock) while the catheter is not in use (27). We found antimicrobial catheter lock solutions with active agents such as trimethoprim/sulfamethoxazole or minocycline to be effective against Nocardia biofilm. Use of these solutions might be a valid way to prevent Nocardia CLABSIs and salvage the CVC, particularly in cancer patients with CLABSI, for whom removal of the CVC might not be possible because of severe thrombocytopenia or lack of other vascular access.
Although no complete correlation exists between in vitro susceptibility and clinical outcome, antimicrobial drug susceptibility tests should be performed for Nocardia isolates in immunocompromised patients to guide therapy. Drug susceptibility of Nocardia spp. varies among species (22).
In agreement with previous results, our findings also confirmed in vitro activity of trimethoprim/sulfamethoxazole, amikacin, and linezolid against Nocardia spp (17,28–31). However, in our study, N. nova complex was characterized by its susceptibility to clarithromycin and resistance to amoxicillin and clavulanate. This distinctive characteristic of N. nova complex is associated with the presence of membrane-bound penicillinase inducible by clavulinic acid (17,27). N. veterana had an antimicrobial drug susceptibility profile similar to that of N. nova complex. No minocycline resistance was observed, but 45% of isolates had intermediate susceptibility MICs, as has been reported (32–34).
CVC removal, along with the use of a combination of antimicrobial agents guided by antimicrobial drug susceptibility, should be the cornerstone of treatment of Nocardia bacteremia, particularly CLABSIs. We recommend using a combination of amikacin, carbapenems, and trimethoprim/sulfamethoxazole until the Nocardia isolate and its antimicrobial drug susceptibility can be determined (17,32,33).
In conclusion, Nocardia bacteria promote heavy biofilm formation in CVCs, and trimethoprim and minocycline in combination with anticoagulants as lock solutions have potent activity against Nocardia biofilm formation. In this study, N. nova complex isolates were the leading cause of Nocardia bacteremia. Isolation of Nocardia bacteria from the blood should always prompt consideration of Nocardia CLABSIs in cancer patients with indwelling CVCs, especially in the absence of signs and symptoms of pneumonia or disseminated infection.
Dr Al Akhrass recently completed an infectious disease fellowship at The University of Texas MD Anderson Cancer Center and is board certified in internal medicine. His research interests include catheter-related infections and biomarkers and infection.
References
- Brown JM, McNeil M. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: L Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology, 8th ed. Washington: American Society for Microbiology; 2003. p. 370–98.
- Beaman BL, Beaman L. Nocardia species: host–parasite relationships. Clin Microbiol Rev. 1994;7:213–64.PubMedGoogle Scholar
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417.PubMedGoogle Scholar
- Kontoyiannis DP, Ruoff K, Hooper DC. Nocardia bacteremia. Report of 4 cases and review of the literature. Medicine (Baltimore). 1998;77:255–67. DOIPubMedGoogle Scholar
- Lui WY, Lee AC, Que TL. Central venous catheter–associated Nocardia bacteremia. Clin Infect Dis. 2001;33:1613–4. DOIPubMedGoogle Scholar
- Torres HA, Reddy BT, Raad II, Tarrand J, Bodey GP, Hanna HA, Nocardiosis in cancer patients. Medicine (Baltimore). 2002;81:388–97. DOIPubMedGoogle Scholar
- Kontoyiannis DP, Jacobson KL, Whimbey EE, Rolston KV, Raad II. Central venous catheter–associated Nocardia bacteremia: an unusual manifestation of nocardiosis. Clin Infect Dis. 2000;31:617–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Central line–associated bloodstream infection (CLABSI) event [cited 2011 Jun 1]. http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Clinical practice guidelines for the diagnosis and management of intravascular catheter–related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. DOIPubMedGoogle Scholar
- Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008;14:908–34. DOIPubMedGoogle Scholar
- Steingrube VA, Brown BA, Gibson JL, Wilson RW, Brown J, Blacklock Z, DNA amplification and restriction endonuclease analysis for differentiation of 12 species and taxa of Nocardia, including recognition of four new taxa within the Nocardia asteroides complex. J Clin Microbiol. 1995;33:3096–101.PubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Susceptibility testing of Mycobacteria, Nocardiae, and other aerobic actinomycetes; approved standard. NCCLS document M24-A. Wayne (PA): The Institute; 2003.
- Hanna H, Bahna P, Reitzel R, Dvorak T, Chaiban G, Hachem R, Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrob Agents Chemother. 2006;50:3283–8. DOIPubMedGoogle Scholar
- Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother. 2007;51:78–83. DOIPubMedGoogle Scholar
- Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008;3:1909–24. DOIPubMedGoogle Scholar
- Wallace RJ Jr, Tsukamura M, Brown BA, Brown J, Steingrube VA, Zhang YS, Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J Clin Microbiol. 1990;28:2726–32.PubMedGoogle Scholar
- Conville PS, Brown JM, Steigerwalt AG, Lee JW, Byrer DE, Anderson VL, Nocardia veterana as a pathogen in North American patients. J Clin Microbiol. 2003;41:2560–8. DOIPubMedGoogle Scholar
- Roth A, Andrees S, Kroppenstedt RM, Harmsen D, Mauch H. Phylogeny of the genus Nocardia based on reassessed 16S rRNA gene sequences reveals underspeciation and division of strains classified as Nocardia asteroides into three established species and two unnamed taxons. J Clin Microbiol. 2003;41:851–6. DOIPubMedGoogle Scholar
- Wallace RJ Jr, Brown BA, Tsukamura M, Brown JM, Onyi GO. Clinical and laboratory features of Nocardia nova. J Clin Microbiol. 1991;29:2407–11.PubMedGoogle Scholar
- Tuo MH, Tsai YH, Tseng HK, Wang WS, Liu CP, Lee CM. Clinical experiences of pulmonary and bloodstream nocardiosis in two tertiary care hospitals in northern Taiwan, 2000–2004. J Microbiol Immunol Infect. 2008;41:130–6.PubMedGoogle Scholar
- Lai CH, Chi CY, Chen HP, Lai CJ, Fung CP, Liu CY. Port-A catheter-associated Nocardia bacteremia detected by gallium inflammation scan: a case report and literature review. Scand J Infect Dis. 2004;36:775–7.PubMedGoogle Scholar
- Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–501. DOIPubMedGoogle Scholar
- Benoit JL, Carandang G, Sitrin M, Arnow PM. Intraluminal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin Infect Dis. 1995;21:1286–8. DOIPubMedGoogle Scholar
- Koldehoff M, Zakrzewski JL. Taurolidine is effective in the treatment of central venous catheter–related bloodstream infections in cancer patients. Int J Antimicrob Agents. 2004;24:491–5. DOIPubMedGoogle Scholar
- Raad I, Buzaid A, Rhyne J, Hachem R, Darouiche R, Safar H, Minocycline and ethylenediaminetetraacetate for the prevention of recurrent vascular catheter infections. Clin Infect Dis. 1997;25:149–51. DOIPubMedGoogle Scholar
- Root JL, McIntyre OR, Jacobs NJ, Daghlian CP. Inhibitory effect of disodium EDTA upon the growth of Staphylococcus epidermidis in vitro: relation to infection prophylaxis of Hickman catheters. Antimicrob Agents Chemother. 1988;32:1627–31.PubMedGoogle Scholar
- Biehle JR, Cavalieri SJ, Saubolle MA, Getsinger LJ. Comparative evaluation of the E test for susceptibility testing of Nocardia species. Diagn Microbiol Infect Dis. 1994;19:101–10. DOIPubMedGoogle Scholar
- Ambaye A, Kohner PC, Wollan PC, Roberts KL, Roberts GD, Cockerill FR III. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol. 1997;35:847–52.PubMedGoogle Scholar
- Gomez-Flores A, Welsh O, Said-Fernandez S, Lozano-Garza G, Tavarez-Alejandro RE, Vera-Cabrera L. In vitro and in vivo activities of antimicrobials against Nocardia brasiliensis. Antimicrob Agents Chemother. 2004;48:832–7. DOIPubMedGoogle Scholar
- Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW, Wallace RJ Jr. In vitro activities of linezolid against multiple Nocardia species. Antimicrob Agents Chemother. 2001;45:1295–7. DOIPubMedGoogle Scholar
- Glupczynski Y, Berhin C, Janssens M, Wauters G. Determination of antimicrobial susceptibility patterns of Nocardia spp. from clinical specimens by Etest. Clin Microbiol Infect. 2006;12:905–12. DOIPubMedGoogle Scholar
- Wallace RJ Jr, Steele LC, Sumter G, Smith JM. Antimicrobial susceptibility patterns of Nocardia asteroides. Antimicrob Agents Chemother. 1988;32:1776–9.PubMedGoogle Scholar
- Tomlin P, Sand C, Rennie RP. Evaluation of E test, disk diffusion and broth microdilution to establish tentative quality control limits and review susceptibility breakpoints for two aerobic actinomycetes. Diagn Microbiol Infect Dis. 2001;40:179–86. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 9—September 2011
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Issam Raad, Infection Control and Employee Health, Unit 1460, Department of Infectious Diseases, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030, USA
Top