Volume 18, Number 6—June 2012
Dispatch
Wild Boars as Hosts of Human-Pathogenic Anaplasma phagocytophilum Variants
Cite This Article
Citation for Media
Abstract
To investigate the potential of wild boars to host Anaplasma phagocytophilum, we analyzed bacterial 16S rRNA and ank genes. DNA sequencing identified several A. phagocytophilum variants, including a predominance of strains known to cause human disease. Boars are thus hosts for A. phagocytophilum, notably, strains associated with human granulocytic anaplasmosis.
The enzootic cycle(s) of Anaplasma phagocytophilum, a tick-transmitted bacterium that causes granulocytic anaplasmosis (GA) in humans (HGA) and certain domesticated animals is driven by the distribution of its vector ticks and wild mammal reservoirs (1). Molecular and phylogenetic analyses of A. phagocytophilum sequences from ticks and hosts provide evidence that this bacterium comprises a complex of closely related strains that differ in their host preferences and pathogenicity (2–4). Although 16S rRNA, groESL, and ank gene variants from horses with GA in Europe, and less frequently from infected dogs, are identical to sequences from most HGA patients (4–7), the wild reservoir hosts for strains causing human anaplasmosis (AP-ha) in Europe are poorly understood.
In contrast to the eastern United States, where white-footed mice are a primary reservoir for strains that infect humans, rodents in Europe have not been found to display high zoonotic potential (8). Moreover, cervids have been found to propagate mostly A. phagocytophilum variants that have not been detected in humans (9,10). An exception to this finding is that red deer seem to maintain strains that induce HGA (4). In Slovenia, identical A. phagocytophilum groESL sequences have been identified in patients and wild boars (Sus scrofa), which suggests that boars may represent a potential reservoir for AP-ha variants (10,11). Although several clinical cases of HGA have been reported in Poland (12), no data are available concerning A. phagocytophilum infections in boars, even though they are the most abundant big game animals (≈200,000 animals are hunted and killed annually) and host all 3 parasitic stages of the tick vector Ixodes ricinus. Thus, we sought to determine the frequency of A. phagocytophilum in populations of wild boars and in host-derived ticks to clarify the role of boars in the ecology and epidemiology of GA.
Sampling was performed at 2 tagging stations, Zielonka and Kąty, in distinct forested areas situated within the Zielonka Primeval Forest, in west-central Poland. EDTA-blood specimens were collected from 325 animals harvested during May–December 2006, 2007, and 2008. The animals represented 3 age groups: piglets (34%), yearlings (49%; >1 to 2 years), and adults (17%). Paired samples of liver and blood were collected from 24 boars. During May–November 2006, 50 animals were inspected for ticks at Zielonka. DNA was extracted from blood and ticks by using Genomic Mini AX Blood and Sherlock AX kits (A&A Biotechnology, Gdynia, Poland). Nested PCR targeting a 546-bp fragment of the A. phagocytophilum 16S rRNA gene was performed (13). Selected positive samples were subjected to a second PCR targeting a 444-bp region of the ankA gene (14).
Selected 16S rRNA and ankA PCR amplicons were sequenced with an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) and analyzed by using BLASTn (www.ncbi.nlm.nih.gov.blast) analysis of GenBank sequences. Phylogenetic dendrograms were constructed by the neighbor-joining algorithm method (Vector NTI Advance version 10.3.0, Invitrogen Corp., Carlsbad, CA, USA). Nine partial 16S rRNA and 6 ankA sequences detected were deposited in GenBank under accession nos. GU391312–GU391320 (7 from boars, 2 from ticks) and GU434664–GU434669 (4 from boars, 2 from ticks), respectively.
Of the 325 animals tested, 39 (12%) yielded A. phagocytophilum 16S rRNA amplification products (Table). Bacteremic hosts were detected in all 3 years with the highest prevalence (20.3%) recorded in 2006 and the lowest (8.8%; χ2 test, p = 0.015) in 2008. Bacterial DNA was identified in all 3 host-age groups, i.e., in 13.6% of piglets, 12.6% of yearlings, and in 7.1% of adults. The overall infection prevalence among animals from Zielonka (17.4%) was significantly higher than among those from Kąty (7.1%; p = 0.004). The finding that 26% of the 50 animals harvested at Zielonka in 2006 were infected represents the highest A. phagocytophilum infection prevalence ever recorded among boars. Of 24 liver samples, 2 (8.3%) tested positive. Both animals with positive liver samples also yielded the bacterium in blood. The remaining 22 blood samples were PCR negative.
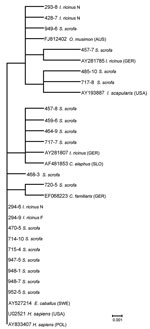
Seventy partially engorged I. ricinus ticks (58 nymphs, 11 females, 1 larva) were collected from 9 animals (7.8 ticks per infested animal). Because all of these ticks parasitized boars with negative blood specimens, the pathogen identified in 3 (5.2%) nymphs and 4 (36.4%) female ticks could have been acquired during their previous blood meal. On the other hand, detecting the same 16S rRNA variant in a female tick (294–9) and in a nymph (294–6; Figure 1) that fed side by side on a yearling, may indicate that the bacterium was acquired by co-feeding transmission (between infected and noninfected ticks).
Sequencing of 16S rRNA products from 27 selected animals (20 from Zielonka, 7 from Kąty) produced 29 sequences (27 from blood, 2 from liver). These sequences showed marked diversity, representing 7 different A. phagocytophilum variants that were 99.6% similar to each other. The most common variant comprised 13 (44.8%) sequences (e.g., 470–5, Figure 1) that matched sequences reported from HGA patients in North America and Europe, including a sequence from a patient in Poland (Figure 1). These sequences, which were related to sequences found in HGA case-patients, prevailed among infected animals (12 of 20) from Zielonka, which harbored them in all 3 years. The second most frequently amplified variant comprised 10 (34.5%) sequences identical to a sequence from red deer in Slovenia. Two hosts from Kąty yielded an AP-variant 1 strain for which white-tailed deer are reservoirs in the United States (15). Three distinct sequences matched sequences from a dog, a tick, and a mouflon in Germany, whereas 1 sequence (468–3) was unique. Two boars with positive liver samples (457; 717, Figure 1) had dual infections caused by distinct variants identified in blood and liver samples, respectively.
Among the 4 sequences from ticks, 2 clustered with the AP-ha variant. Twelve partial ankA sequences detected in 10 boars and 2 ticks showed 98.9% homology to each other. Seven (70%) animals yielded sequences (e.g., 470–5; Figure 2) identical to a sequence from an HGA patient in Sweden. Their corresponding 16S rRNA gene sequences clustered with the AP-ha variant. Of the remaining ankA sequences, 1 matched a sequence from a roe deer in Poland, whereas 2 were unique. These 3 sequences were identical on the basis of the 16S rRNA gene. This finding confirms that the ankA gene is a more informative marker for the characterization of genetic diversity in A. phagocytophylum (4). Among 2 ankA sequences obtained from ticks, a female tick yielded the predominant ankA variant, whereas a nymph harbored a novel variant.
The presence of bacteremic animals (range 9%–20%) throughout this study provides compelling evidence for the involvement of wild boars in the enzootic cycle of A. phagocytophilum. Further studies with larger tick samples are necessary to investigate the efficiency of boar-to-tick transmission. The fact that most of the partial 16S rRNA and ankA sequences (13 of 27 and 7 of 10, respectively) amplified from boars corresponded to A. phagocytophilum strains known to cause human disease, reconfirms earlier findings in which groESL sequences identical to those from patients in Slovenia were found in wild boar (S. scrofa) populations in the Czech Republic and Slovenia (10,11). Detection of A. phagocytophilum strains associated with human infections in all 3 boar age groups in Zielonka, as well as in host-derived ticks, strongly implicates the wild boar as a notable host of HGA variants.
Because bacteremia among hosts from Zielonka was frequent (range 12%–26%) and boars are quite abundant in Europe, they could be used as sentinel animals for detecting A. phagocytophilum infections, notably strains known to be infectious for humans. Additional molecular and serologic studies including essential reservoir-competence experiments with AP-ha strains are needed to further elucidate the role of wild boars in the epidemiology of A. phagocytophilum.
Dr Michalik is a researcher in the Department of Animal Morphology at Adam Mickiewicz University in Poznań. His research focuses on host-vector-pathogen relationships, with a particular emphasis on the ecology of tickborne diseases.
Acknowledgments
We thank Robert S. Lane for valuable comments on the manuscript.
This research was funded by the State Committee for Scientific Research (grant no. 2PO4C 111 29).
References
- Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. 2010;167:108–22. DOIPubMedGoogle Scholar
- Massung RF, Mauel MJ, Owens JH, Allan N, Courtney JW, Stafford KC III, Genetic variants of Ehrlichia phagocytophila, Rhode island and Connecticut. Emerg Infect Dis. 2002;8:467–72. DOIPubMedGoogle Scholar
- de la Fuente J, Massung RF, Wong SJ, Chu FK, Lutz H, Meli M, Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. J Clin Microbiol. 2005;43:1309–17. DOIPubMedGoogle Scholar
- Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. 2011;49:790–6. DOIPubMedGoogle Scholar
- Johansson K-E, Pettersson B, Uhle’n M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. Res Vet Sci. 1995;58:109–12. DOIPubMedGoogle Scholar
- Strasek Smrdel K, Tozon N, Duh D, Petrovec M, Avsic Zupanc T. Diversity of groESL sequences of Anaplasma phagocytophilum among dogs in Slovenia. Clin Microbiol Infect. 2009;15(Suppl 2):79–80. DOIPubMedGoogle Scholar
- von Loewenich FD, Stumpf G, Buamgarten BU, Rollinghoff M, Dumler JS, Bogdan C. A case of equine granulocytic ehrlichiosis provides molecular evidence for the presence of pathogenic Anaplasma phagocytophilum (HGE agent) in Germany. Eur J Clin Microbiol Infect Dis. 2003;22:303–5 .DOIPubMedGoogle Scholar
- Liz JS, Anderes L, Sumner JW. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–7.PubMedGoogle Scholar
- Petrovec M, Bidovec A, Avsic-Zupanc T, Koren S, Sumner JW, Nocholson WL, Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien Klin Wochenschr. 2002;114:641–7.PubMedGoogle Scholar
- Petrovec M, Sixl W, Schweiger R, Mikulasek S, Elke L, Wust G, Infections of wild animals with Anaplasma phagocytophila in Austria and the Czech Republic. Ann N Y Acad Sci. 2003;990:103–6. DOIPubMedGoogle Scholar
- Strasek Smrdel K, Bidovec A, Malovrh T, Petrovec M, Duh D, Avsic Zupanc T. Detection of Anaplasma phagocytophilum in wild boar in Slovenia. Clin Microbiol Infect. 2009;15(Suppl 2):50–2. DOIPubMedGoogle Scholar
- Grzeszczuk A, Puzanowska B, Zirako S. Anaplasma phagocytophilum infection in patients with early Lyme borreliosis, erythema migrans, in north-eastern Poland. Clin Microbiol Infect. 2009;15(Suppl 2):17–8. DOIPubMedGoogle Scholar
- Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, A nested PCR assay for the detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–5.PubMedGoogle Scholar
- Walls JJ, Caturegli P, Bakken JS, Asanovich KM, Dumler JS. Improved sensitivity of PCR for diagnosis of human granulocytic ehrlichiosis using epank1 genes of Ehrlichia phagocytophila–group ehrlichiae. J Clin Microbiol. 2000;38:354–6.PubMedGoogle Scholar
- Massung RF, Courtney JW, Hirtzak SL, Pitzer VE, Smith G, Dryden R. L. Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis. 2005;11:1604–6. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 18, Number 6—June 2012
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence Jerzy Michalik, Department of Animal Morphology, Faculty of Biology, Adam Mickiewicz University, ul. Umultowska 89, 61-701 Poznań, Poland
Top